This article is a summary of "Single-stage chromatographic clarification of Chinese Hamster Ovary cell harvest reduces cost of protein production" by researchers from Ambrx, Inc. and 3M. protein production", please refer to the original article for details.
A single-stage clarification was developed using a disposable chromatographic clarification device (CCD) to recover recombinant proteins from Chinese hamster ovary (CHO) harvested cell culture fluid (HCCF). Clarification of CHO HCCF is a complex and expensive process involving multiple centrifugation and/or deep filtration steps to remove cells and debris and reduce process-related impurities such as host cell proteins (HCP), nucleic acids, and lipids. When using deep filtration, the filter chain consists of a plurality of filters with different ratios, number of layers, pore sizes, and adsorption characteristics. Deep filters combined with 0.2 micron membrane filters clarify HCCF based on size exclusion, adsorption, and charge-based mechanisms and provide robust bioburden control. Each stage of the clarification process requires time, labor and utilities, with product loss at each step. Here, the use of the 3M Harvest RC Chromatographic Clarifier, a single-stage CCD, was identified as an alternative strategy to the three-stage filtration series. The CCD results in a smaller overall filtration area, smaller rinse volume, and higher yields. The single-stage clarification process was compared to a tertiary filtration process using a bioprocess cost model. By compressing CHO HCCF clarification into a single-stage cascade, the total cost of the clarification process was reduced by 17-30%, depending on bioreactor size. The primary drivers of cost reduction were reductions in total filtration area, labor, time, and utilities. The advantages of the single-stage harvesting process extend throughout the downstream process, resulting in a 25% relative increase in cumulative yield and comparable impurity removal.
Therapeutic proteins, including monoclonal antibodies (mAb), bispecific antibodies, cytokines, and enzymes, are typically produced using a replenishment batch or perfusion mammalian cell culture process in which the target proteins are expressed and secreted by cells. Target proteins are recovered from harvested cell culture fluids (HCCF) using centrifugation and/or deep filtration as the primary recovery step to remove cellular and cellular debris and to reduce process-associated impurities such as host cell proteins, nucleic acids, and lipids.
The use of centrifugation or deep filtration is a product-specific decision in which factors such as size and clinical stage of production (e.g., Phase I or commercialization), capital equipment, cost, cleanability, and facilities (dedicated or multi-product) are considered. The development of deep filtration unit operations is less complex compared to continuous flow centrifugation, which translates into predictable scale-up and ease of use. Multi-product facilities that support early production, such as contract development and manufacturing organizations (CDMOs), rely on disposable deep filtration as the primary clarification step, ultimately increasing facility flexibility, capacity, and productivity. In addition, disposable deep filtration significantly reduces cleaning validation requirements and facility downtime due to product changeover compared to centrifugation.
During the development of a Phase 1 clinical manufacturing process for a recombinant non-antibody protein product expressed in Chinese hamster ovary (CHO) cells, a baseline, two-stage deep filtration process was developed to recover the target proteins from cell culture fluids (CCF) (Figure 1A). After a series of orthogonal chromatographic separations including cation exchange (CEX), mixed-mode anion exchange (MAX), mixed-mode exchange (MCX), polyethylene glycolization, and further purification, the level of residual host cell proteins (HCPs) in the drug substrate (DS) was approximately 200 ng HCP/mg product. Other HCP reduction strategies were explored using precipitation aids, wash buffer additions, and packing screens (hydrophobic interaction chromatography (HIC), anion exchange (AEX), cation exchange (CEX), and mixed-mode (MM)), which resulted in little further HCP reduction.
A disposable clarification device based on AEX fiber chromatography (3M Emphaze AEX Hybrid Purifier) was introduced as the third stage in the HCCF clarification series, resulting in an additional 50% reduction in HCP levels (Figure 1B). The primary, secondary, and tertiary clarification stages were operated in tandem with a frontal surface area ratio of 3:3:1. After purification and polyethylene glycolization, HCP levels measured in the drug substance (DS) were approximately 10 ng HCP/mg of product, demonstrating that the chromatographic clarification stage provided an additional 1.3 LRV relative to the two-stage deep filtration series.
Recently, a new solution has emerged that uses fiber chromatography to efficiently and scalably remove cells, cellular debris, and key soluble contaminants. The 3M Harvest RC Chromatographic Clarifier is a single-stage chromatographic clarification device (CCD) consisting of a bed of AEX quaternary ammonium (Q) ligand-functionalized polypropylene fibers and a 0.2 um membrane. During cell culture clarification, cells and cellular debris electrostatically bind to the AEX fibers, effectively retaining large and small particles without forming a surface cake layer. The charged fiber media also effectively removes impurities associated with soluble processes (i.e., host cell proteins and nucleic acids), resulting in a cleaner effluent than centrifugation or conventional deep filtration. The recommended operating differential pressure for CCD is ≤5 psi, which reduces cell shearing and lysis, further improving the quality of the HCCF and minimizing the potential for disulfide reduction. In addition, CCD's synthetic AEX media requires less rinsing prior to use than traditional wet deep filtration media, saving utilities and time. In addition, water is used as the CCD rinse solution, thus eliminating the need for buffer preparation and associated buffer batch record quality release.
Considering the effective clarification and removal of HCP and DNA using a tertiary chain, single-stage clarification was developed as a direct alternative, utilizing the physical and electrostatic properties of the CCD to effectively remove cells, HCP, and DNA (Figure 1C). Based on these results, the total filter area required for the cell culture clarification step was reduced by a factor of 2.5. Purification and polyethylene glycolization resulted in an approximate 25% increase in throughput for the entire process, while all product quality attributes met acceptance criteria and were comparable to a three-stage filter chain.
Production costs for the cell culture clarification step were modeled to assess costs and savings using all relevant inputs for a three-stage versus a single-stage clarification approach. By compressing CHO cell culture clarification to one stage, the total cost of the clarification process was reduced by 17%-30%, depending on size (e.g., bioreactor volume). The primary drivers of cost reduction were reductions in total filter area, labor, and utility usage. With the implementation of a single-stage chromatographic clarification process, the total time for cell culture clarification, including preparation (fiber bed rinsing and equilibration), product filtration, and washing, was reduced by approximately 50%.
Please refer to the original article for detailed test operations, results and discussion.
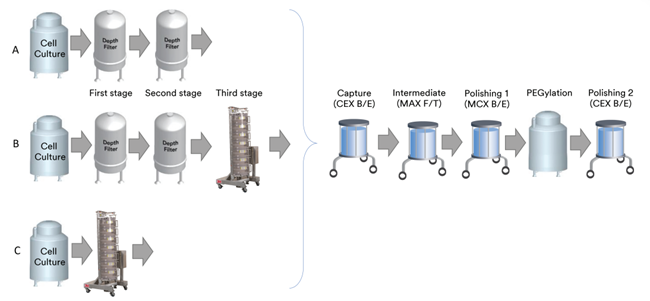
Figure 1. Process flow for the production of non-antibody protein products. (a) two-stage deep filtration using 3M Zeta Plus Filter Capsules 10SPO2A and 90ZBO5A, (b) three-stage filtration using Zeta Plus Filter Capsules 10SPO2A and 9OZBO5A and a 3M Emphaze AEX Hybrid Purifier. and (C) single-stage chromatographic clarification using a 3M Harvest RC Chromatographic Clarifier. After clarification, all chains were processed by the same downstream purification unit operations: binding-eluting cation exchange chromatography (BECEC), flow-through mixed-mode anion exchange chromatography (FCMEC), binding-eluting mixed-mode cation exchange chromatography (BECEC), polyethylene glycolization (PEG), and binding-eluting cation exchange chromatography (BEC). eluting cation exchange chromatography.
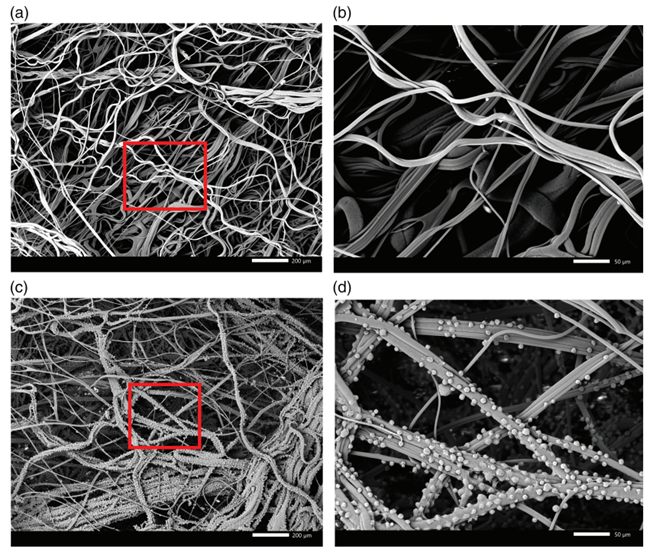
Figure 2. Scanning electron microscope (SEM) images of 3M Harvest RC Chromatographic Clarifier after SEM preparation steps (e.g., fixation, dehydration, critical point drying, and gold sputter coating). The fiber media layer was extracted from the device before clarification (a, b) and after HCCF clarification (c, d). Red boxes in (a) and (c) indicate magnified images of (b) and (d), respectively.
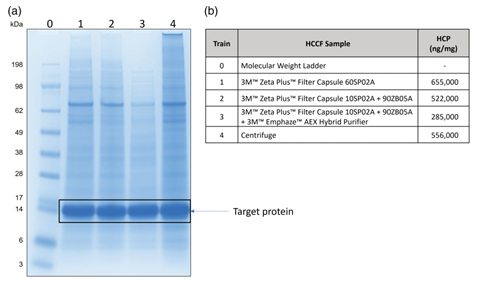
Figure 3. (a) Non-reducing SDS-PAGE of chain 1-4 HCCF. (b) Host cell protein (HCP) analysis of chain 1-4 HCCF.
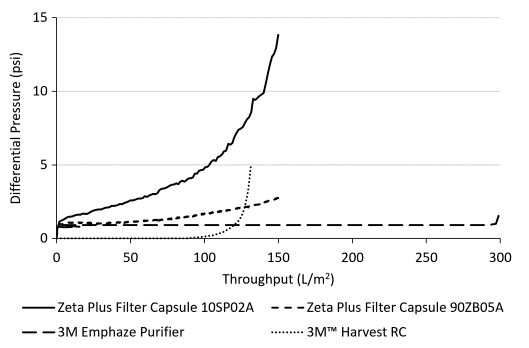
Figure 4. Differential pressure curves for three-stage filtration (Chain 3) and single-stage chromatographic clarification using the 3M Harvest RC Chromatographic Clarifier (Chain 5).
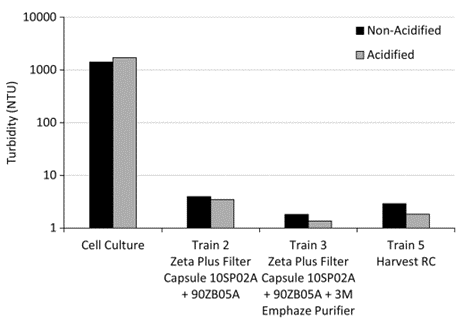
Figure 5. Turbidity (NTU) values (unacidified and acidified) of cell culture starting materials for chains 2, 3 and 5 and of clarified filtrate samples.
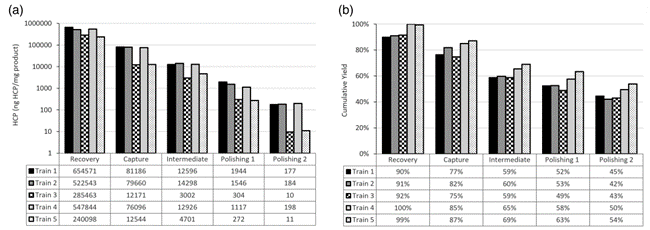
Figure 6. (a) Host cell protein (HCP) levels and (b) cumulative yield of process intermediates from different harvesting strategies (chains 1-5).
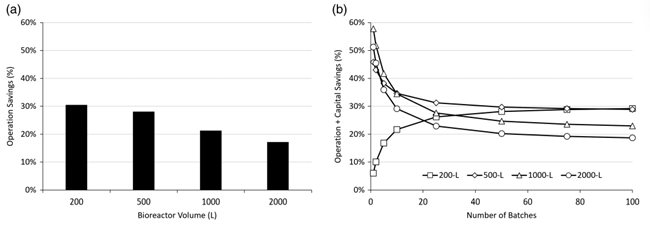
Figure 7. (a) Percentage savings in operating costs (filters, labor, water, and piping) for single-stage clarification (Chain 5) compared to three-stage clarification (Chain 3) for different bioreactor volumes in a single run. (b) Total cost savings, including capital equipment (operating costs and filter holders), versus number of batches at different bioreactor volumes.
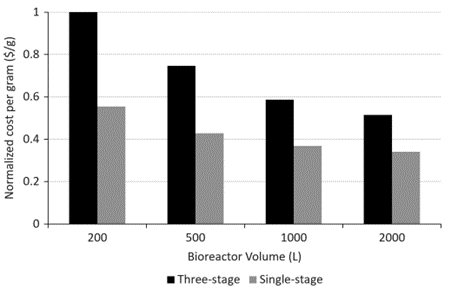
Figure 8. Normalized cost per gram of purified API for single-stage and tertiary clarification processes at different bioreactor volumes (data normalized to 200 L of tertiary clarification).
Summarize
Compressing clarification unit operations into a single-stage CCD reduces the cost of cell culture clarification steps, reduces process-related impurities (HCP and DNA), and increases productivity. Operating cost savings are reduced by 17-30% per run, depending on bioreactor size. Taking into account the cost of capital equipment, overall cost savings after 100 batches were 29% and 19% for the 200 L and 2000 L bioreactors, respectively. Increased HCP reduction during the cell culture clarification phase (Chain 3 and Chain 5) reduces the burden on the chromatography unit operation and translates into lower HCP levels at the end of the process. Chromatographic clarification (Chain 3 and Chain 5) resulted in HCP levels (10 ng HCP/mg product) that were fully within specification (below 100 ng HCP/mg product) for both processes. By intensifying the cell culture clarification step, the overall relative yield increased by 25% compared to the tertiary process. As a result, the cost of the clarification step per gram of purified API was reduced by 40%. Single-stage CCDs have been successfully scaled up for GMP production and have produced yields and HCP levels comparable to laboratory scale.
original text:B.O'Mara, N.K.Singh, A. Menendez., Single-stage chromatographic clarification of Chinese Hamster Ovary cell harvest reduces cost of protein production. Biotechnology Progress, 2023.






