Vaccination is undoubtedly one of the most dramatic health achievements in human history. In just over two centuries, vaccines have enabled us to achieve extraordinary goals, such as the complete eradication of smallpox, the elimination of polio from much of the world and the dramatic reduction of mortality and morbidity from many infectious diseases.
Vaccination policies are a cornerstone of public health, and great importance is attached to ensuring that safe and effective vaccines are administered. The effectiveness of a vaccine depends not only on the antigenic components, but also on the adjuvants that are often used to stimulate the immune system in a more effective way. Adjuvants are components added to vaccines to improve the immune response to antigens. In addition, adjuvants have several benefits, such as allowing a reduction in the amount of antigen per dose of vaccine and in the number of vaccinations; in some cases they also increase the stability of the antigenic components, prolonging their half-life and indirectly improving their immunogenicity.
Antigens are associated with adjuvants and they are able to induce a local pro-inflammatory response by activating the innate immune system, leading to recruitment of immune cells to the injection site. Antigen-adjuvant complexes activate the pattern recognition receptor (PRR) pathway through pathogen-associated molecular patterns (PAMP). This leads to activation of innate immune cells that produce cytokines and chemokines.
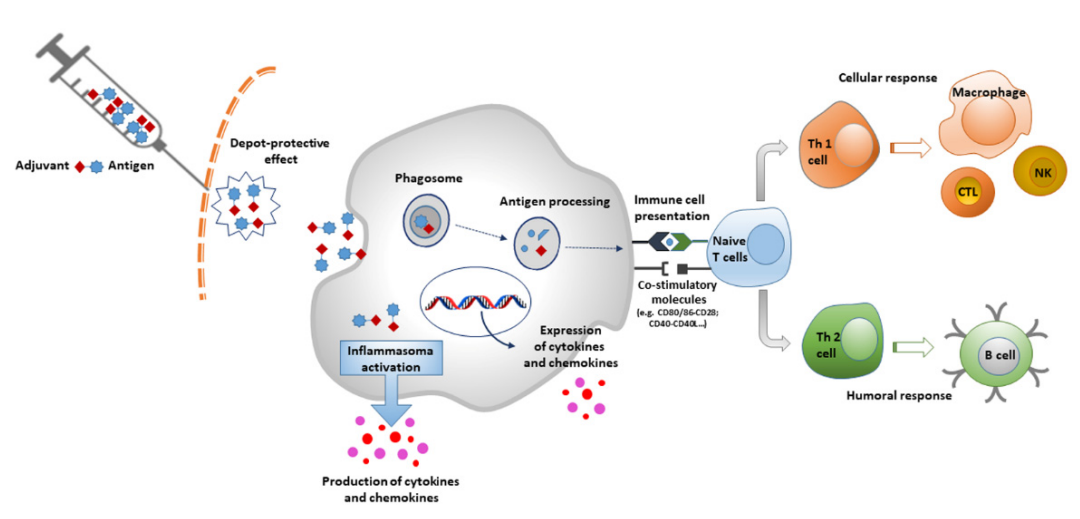
Currently, the vast majority of vaccines approved for human use by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) use aluminum salts as adjuvants, which are the oldest adjuvants used in vaccine formulations. In order to improve the safety and efficacy of vaccines, it is essential to increase the variety and number of new adjuvants. Advances in modern technologies such as nanotechnology and molecular biology have strongly facilitated the process of developing adjuvant components to improve the effectiveness of vaccines. A good adjuvant must be safe, effective and easy to produce; have good medicinal properties (pH, osmolality, endotoxin levels, etc.) and durability; and finally, be economically affordable. Microparticles, emulsions, and immunostimulants show great potential for vaccine production.
Adjuvants can be categorized according to different criteria, such as their physico-chemical properties, origin and mechanism of action. One of the most popular classification systems is based on their mechanism of action, which is divided into two broad categories: delivery system adjuvants and immune-enhancing adjuvants.
aluminum salt
The adjuvant properties of aluminum salts were discovered in the 1920s, and since 1926 these compounds have been used as vaccine adjuvants. As the longest-used adjuvant and the most frequently used in vaccines, approximately one third of currently licensed vaccines contain aluminum adjuvants.
In vaccines, aluminum exists as a complex polymer of crystalline aluminum hydroxide (AlH) or amorphous aluminum hydroxyphosphate (AlP), which forms aggregated nanoparticles.AlH has the appearance of acicular nanoparticles, whereas AlP presents as a reticulum when viewed under a transmission electron microscope. Both forms of aluminum adjuvant are usually soluble in citrate, but AlP is more soluble than AlH. The antigen adsorbs to the surface of the adjuvant particles by electrostatic interactions and ligand exchange. Aluminum salt/antigen binding enhances antigen uptake and presentation by antigen-presenting cells (APCs). In addition, aluminum salts stimulate the activation of NLRP3 inflammatory vesicles, leading to the production of IL-1β and IL-18, which causes local inflammation and APC recruitment.
Aluminum adjuvants are used in many vaccines, such as those against diphtheria and tetanus, whooping cough, hepatitis B, and pneumococcus and meningococcus. In Europe, the European Pharmacopoeia sets the level of aluminum in vaccines at a maximum of 1.25 mg per dose, and in the United States of America, the U.S. Code of Federal Regulations (CFR) sets the level of aluminum in biological products, including vaccines, at 0.85 mg per dose. Unlike aluminum, which is primarily found in food in the form of soluble citrate or chloride salts, inorganic aluminum compounds used as adjuvants are less soluble; this is part of their secondary mode of action. Consequently, due to this poor solubility at physiological pH, the rate of absorption of the aluminum contained in the vaccine after intramuscular or subcutaneous injection will be very slow.
Several studies have evaluated the kinetics of aluminum after intramuscular injection. One experiment investigated AlH and AlP based on intramuscular injection of AlH and AlP labeled with 26Al at a total dose of 0.85 mg of aluminum.The results showed that the absorption of AlH and AlP was 17% and 51%, respectively, over the 28 days of the experiment.The maximal serum concentration (Cmax) of 26Al was 2 μg/L.Additionally, the neurotoxicity of aluminum has been studied in vitro, in isolation and in animal models, and in humans. studies have been conducted. Some in vitro studies with bacteria have shown no mutagenicity. In contrast, the results of some in vivo studies are often inconsistent and contradictory, and there may be methodological flaws. Thus, to date, it has not been possible to determine whether aluminum salts used as adjuvants (at the recommended doses) produce toxic effects, despite the fact that they produce more or less intense oxidative stress.
Fuchs' adjuvant
Freund's adjuvants include complete and incomplete Freund's adjuvants. Both adjuvants are water-in-oil emulsions that carry antigens and stimulate the innate immune system. Complete Freund's adjuvant (CFA) includes heat-killed mycobacteria in its structure, which enhances the stimulation of the immune response. However, CFA is capable of inducing intense, long-lasting localized inflammation, which can be significantly painful and may result in ulceration at the injection site. Incomplete Freund's adjuvant (IFA), which does not contain Mycobacterium, was used as an adjuvant for the human influenza vaccine in the 1950s; it induces a stronger, longer-lasting antibody response than the same vaccine without adjuvant.
The adjuvant activity of IFA is based on its characterization as an oily antigenic deposit from which antigen is continuously released at the injection site. This leads simultaneously to an increase in antigen half-life and a strong local innate immune stimulation through phagocytosis, leukocyte recruitment and infiltration, and cytokines. However, the routine use of IFA in human vaccine formulations triggers strong side effects. A survey conducted by the World Health Organization in 2005 showed that 40,000 of approximately 1 million IFA subjects immunized experienced serious side effects (e.g., sterile abscesses).
MF59
MF59 is a water-in-oil emulsion consisting of squalene, Span 85 and Tween 80 in 10 mM sodium citrate buffer at pH 6.5, with an average particle size of about 165 nm.This is the first oil-in-water emulsion approved for use in human vaccines in Italy in 1997. It is currently used in the trivalent and quadrivalent (TIV and QIV) influenza vaccine Fluad (Seqirus). Studies have shown that the presence of MF59 improves the effectiveness of influenza vaccines in children under the age of 2. MF59 has also been used as an adjuvant in HBV vaccines, triggering a strong immune response, better than that induced by aluminum.
Regarding the mechanism of action, MF59 has a similar effect to aluminum salts. Reservoir activity at the injection site is negligible, with studies indicating a half-life of 42 hours. In contrast, MF59 has a potent ability to induce cellular and humoral immune responses, including the production of high titers of functional antibodies.The presence of MF59 stimulates local innate immune cells to secrete chemokines, such as CCL4, CCL2, CCL5, and CXCL8, which, in turn, drive leukocyte recruitment, antigen uptake, and migration to lymph nodes by triggering adaptive immune responses.MF59 is safe and well tolerated well, with millions of doses administered in more than 35 countries.
AS03
AS03 is an oil-in-water adjuvant emulsion consisting of the surfactant polysorbate 80 and two biodegradable oils, squalene and DL-alpha-tocopherol in phosphate buffer. This adjuvant has been used in influenza vaccines, triggering an immune response similar to that of MF59, and in malaria vaccines. The European Union approved the marketing of the AS03-adjuvanted vaccine Pandemrix in 2009, while the AS03-adjuvanted influenza A (H5N1) monovalent vaccine was approved by the FDA in 2013.
The antioxidant and immunostimulatory properties of α-tocopherol appear to be stronger compared to MF59. In addition, it has been shown that AS03 is able to stimulate the immune system by activating NF-κB, which induces cytokine and chemokine secretion in muscle and lymph nodes and promotes the migration of innate immune cells.AS03 also stimulates CD4+ T-cell specific immune responses, which can lead to long-lasting neutralizing antibody production and higher levels of memory B-cells.AS02 builds on the AS03 was further supplemented with two powerful immunostimulants, QS-21 (a saponin derived from Astragalus membranaceus) and 3-O-deacyl-4′-monophosphoryl lipid a (MPL), to enhance its immunogenicity.
virus-like particle
Virus-like particles (VLPs) are icosahedral or rod-shaped nanoparticles (Å20-200 nm) consisting of the outer shell of a self-assembled coat protein; they have long been studied for vaccine development. They are non-infectious particles and do not include any genetic material.VLPs are formed from external viral capsids with repetitive epitopes that are immediately recognized by the immune system as non-self, resulting in a robust immune response. In addition to these repetitive structural motifs, VLPs are similar in size to viruses (typically between 20-800 nm) and are processed quickly and efficiently to produce a rapid and long-lasting immune response, even in the absence of adjuvants.
Currently, there are two important vaccines that use virus-like particle adjuvants to induce immunity: the hepatitis B and papillomavirus (HPV) vaccines. The hepatitis B vaccine currently in use is a recombinant DNA vaccine containing hepatitis B surface antigen (HBsAg) in the form of VLP for the prevention of hepatitis B infection, which is administered to infants, children, and adolescents younger than 15 years of age, or to those at high risk for hepatitis B infection, and has also shown good immunogenicity in newborns born to mothers who are carriers of hepatitis B (95-99% in terms of effectiveness).
The HPV vaccine is also based on the VLP platform. The current nine-valent HPV vaccine protects against nine different viral genotypes that cause 90% of cervical cancers and 80-95% of anogenital cancers. The nine-valent HPV vaccine contains the L1 proteins of nine different genotypes of HPV (6, 11, 16, 18, 31, 45, 53, 58), which form the VLP and are synthesized by recombinant DNA technology.
virus body
Virosomes are a vaccine platform that closely resembles the structure of natural viruses. Structurally, they are VLPs formed from a recombinant influenza virus envelope consisting of hemagglutinin (HA), neuraminidase (NA), and phospholipids (phosphatidylethanolamine and phosphatidylcholine) that lack the genetic material of the virus.Virosomes were first used in the manufacture of an influenza vaccine in 1975, and Influenza Virosomes are an adjuvanted influenza vaccine suitable for use in all age groups and are effective in healthy and immunocompromised children, adults and the elderly. It induces a B-cell response and production of specific antibodies. The virosomes retain the receptor-binding capacity and membrane fusion activity of viral HA, but due to the lack of viral RNA, they are unable to induce infection in cells upon binding.
Viroids are a perfect delivery system to transfer antigens into the cytoplasm of antigen-presenting cells and induce cytotoxic T-lymphocyte (CTL) responses. However, due to their weak adjuvant properties, virosomes are not very effective in activating APCs and promoting cross-presentation. This intrinsic limitation can be eliminated by adding stronger adjuvants. For example, a novel virosome-based influenza vaccine has recently been developed that is supplemented with the Toll-like receptor 4 (TLR4) ligand monophosphoryl lipid a (MPLA) and the metal ion chelating lipid DOGS NTA-Ni adsorbed into membranes.In vivo immunization of mice with virosomes adjuvanted with these MPLA adjuvants induces specific CTL.
The significant advantage of adjuvants for virosomal delivery systems is their ability to adsorb antigens to their surfaces and lumens through hydrophobic lipid interactions. Adsorption of antigens onto the fluid phospholipid bilayer surface of virosomes stimulates interactions with host cell receptors.The FDA has approved virosomes as nanocarriers for human use, and they are very well tolerated and safe. In contrast to subunit vaccines, which do not respond well to viral invasion, virosomes are able to induce robust humoral and cellular immunity in a manner very similar to natural infections and other potent adjuvants.
To date, in addition to the two viroid-based vaccines for influenza and hepatitis A mentioned above, several other viroid-based vaccines are under investigation, including vaccines against HIV, HPV, respiratory syncytial virus and malaria.
TLR1/2 agonist
Among TLR1/2 agonists, L-pampo is a potent adjuvant system consisting of Pam3Csk4 (Pam3) and polyinosinic acid:polycytidylic acid (polyI:C). In one study, L-pampo induced stronger anti-HBV antibody production than aluminum adjuvant and also involved cell-mediated immune responses such as increased multifunctional CD4+ T cells.
In addition, bacterial lipoproteins are the most potent ligands recognized by TLR2. Studies have shown that synthetic lipopeptides derived from bacterial lipoproteins are strong activators of B cells and macrophages and can be used as vaccine adjuvants. Macrophage-activated lipoprotein-2 (MALP-2) from Mycoplasma fermentans was shown to activate immune cells through TLR2- and MyD88-dependent signaling pathways. In addition to MALP-2, Pam2CSK4 and Pam3CSK4 are recognized TLR2 agonists that have been evaluated as therapeutic agents against infectious diseases such as Leishmania protozoa, malaria and influenza.
TLR3 agonist
TLR3, an endosomal receptor that detects viral dsRNA, recognizes poly(I:C) because it structurally mimics viral RNA, thereby inducing the production of type I IFN and type III IFN and triggering a Th1 cytokine response.Type I IFN produced after TLR3-poly(I:C) interaction is important for the efficient activation of conventional dendritic cells (cDC) to activate CD8+ T cell responses is particularly important. In addition, poly(I:C)-generated type I IFN stimulates clonal expansion of T cells, increasing the effector T cell ratio and the number of antigen-specific B cells.
poly(I:C) has been extensively studied as a potential adjuvant. However, poly(I:C) has toxic effects on humans. Therefore, scientists' attention has been focused on poly(I:C) derivatives, such as poly(ICLC) and poly(IC12U), as well as other synthetic TLR3 agonists, such as ARNAX, IPH3102, and RGC100.
To date, several studies have used poly(ICLC) as a vaccine candidate for infectious diseases such as Plasmodium falciparum and HIV, as well as for cancer. Studies have shown that poly(ICLC) triggers a stronger Th1 immune response than other TLR agonists such as LPS and CpG. A new TLR3 agonist with adjuvant potential is ARNAX, a TLR3-specific ligand that is less toxic than poly(I:C).Two of the most important areas of ARNAX research are cancer immunotherapy and influenza vaccines.
TLR4 agonist
TLR4 agonists studied as vaccine adjuvants include AS01, AS02, and AS04, all of which contain MPLA, a ligand for endosomal TLR4.AS01 has been used in the development of vaccines against malaria, HIV, and tuberculosis.AS01 is a co-adjuvant system comprised of two different immune stimulating molecules, MPLA and QS-21, encapsulated in a liposomal structure. These two compounds use liposomes as carriers to reach the cell via cholesterol-dependent endocytosis. Intracellularly, QS-21 causes lysosomal destabilization and promotes activation of the protein kinase SYK.MPLA acts on the endosome TLR4 to induce TRIF-dependent signaling pathways.AS01 activates cystatinase-1, which promotes the activation of the NLRP3 inflammatory vesicle as well as the release of IL-1β and IL-18 from the APC.The release of IL-18 leads to IFN-γ rapid production of natural killer cells, especially in lymph nodes, which promotes DC maturation and induction of Th1-type immune responses.
TLR5 agonist
TLR5 is a receptor that recognizes bacterial flagellin and is expressed by several immune cells. The attachment to the ligand leads to the activation of inflammatory pathways and the release of many inflammatory mediators, such as TNF-α, IL-1β, IL-6 and nitric oxide. In addition, flagellin is capable of eliciting both Th1 and Th2 responses, unlike other TLR ligands, which only induce Th1 responses. Flagellin induces IL-1β production and release by activating the NLRC4 inflammasome. To date, at least three vaccines using flagellin as an adjuvant are in clinical trials: two against influenza virus and one against Yersinia pestis.
TLR7/8 agonists
Several studies have shown that agonists of TLR7/8 strongly induce Th1 immune responses. Ligand binding to TLR7/8 is capable of producing high levels of type I IFN, IL-12, TNF-α, and IL-1β. In addition, TLR7/8 and TLR9 agonists are the only agonist molecules capable of activating and promoting the clonal expansion of cDCs and plasmacytoid dendritic cells (pDCs), as well as mobilizing CD14+CD16+ inflammatory monocytes and CD14dimCD16+ patrolling monocytes.
The most representative TLR7/8 agonists are synthetic small molecules such as imiquimod (R837) and raquinimod (R848), which belong to the imidazoquinoline class. Imiquimod is currently approved for the treatment of genital warts, superficial basal cell carcinoma, and actinic keratosis, while raquinimod has been investigated for antiviral and anticancer therapies.
However, these small molecules have some inherent limitations. In particular, they can diffuse away from the site of administration and thus away from the antigen, leading to reduced efficacy and induction of systemic side effects. Direct binding of these molecules to aluminum adjuvants has been shown to enhance vaccine efficacy. In addition, binding to synthetic polymer scaffolds, lipid polymer amphiphiles, polyethylene glycol (PEG), nanogels, alum, and a variety of other synthetic polymers significantly increased imidazoquinoline delivery and improved maturation of DCs and antigen-specific T cells. In addition, previous studies using imidazoquinoline with one or more other TLR agonists (e.g., MPLA and MPLA+CpG ODN) demonstrated that this combination increased innate immune responses, significantly produced antigen-specific neutralizing antibodies, and improved Th1 responses. All of these innovations highlight the superior potential of TLR7/8 agonists as adjuvant candidates.
TLR9 agonist
TLR9 naturally recognizes bacterial DNA motifs represented by unmethylated cytosine phosphoguanine (CpG) dinucleotides and drives activation of the innate immune system through a MyD88-dependent pathway.CpG-ODNs elicit potent chemokine, cytokine, and antibody production in NK cells, B cells, and pDCs, which drives a strong Th1-type immune response. To date, three (A-C) different classes of CpG-ODNs have been developed, but only molecules from class B have been used as adjuvants in clinical trials.Type B CpG-ODNs induce maturation of pDCs and interact directly with B cells to enhance antibody production.
Recently licensed CpG 1018, an oligonucleotide with high chemical stability and the ability to trigger an adjuvant Th1-type immune response, is being used as an adjuvant for the hepatitis B vaccine, Heplisav-B. CpG 1018 in Heplisav-B improves vaccine efficacy, requiring only two doses compared to three doses of the traditional hepatitis B vaccine, which requires three doses for optimal protection. Vaccine. Another CpG-ODN, CpG 7909, is also under clinical evaluation and has shown encouraging results in HBV and malaria vaccination.
In addition, other next-generation TLR9 agonists are in development.MGN1703, is a small DNA molecule that includes CG motifs but is structurally distinct from CPG-ODN.MGN1703 consists of a segment of reverse complementary DNA that is double-stranded in the middle, with single-stranded loops at each end that include three non-methylated CG motifs, forming a dumbbell-shaped structure.MGN1703 has been tested as an adjuvant for cancer vaccines and found to activate both innate and adaptive immune responses with only minor or temporary side effects.
Vaccination has been and will continue to be one of the most powerful weapons in humanity's fight against infectious diseases. Thanks to these effective and safe preventive tools, humankind has been able to eradicate the most formidable enemies in human history from many parts of the world. The recent pandemic of C.pneumoniae has fully emphasized the importance of vaccination, and further progress in vaccine research is necessary to prepare for possible future pandemics. The efficacy of vaccines depends on the essential properties and action of the adjuvants. With regard to the future of vaccination, more attention must be paid to these molecules in order to produce increasingly safe and effective vaccines for the benefit of mankind.






