Summaries
Keywords: vaccination, adjuvants, modern technology, future prospects.
Vaccination is undoubtedly one of the most remarkable health achievements in human history. In just over two centuries, vaccines have enabled us to achieve extraordinary goals, such as the complete eradication of smallpox, the elimination of polio from much of the world, and the dramatic reduction of mortality and morbidity from many infectious diseases in a number of countries. Vaccination policies are the cornerstone of public health in many parts of the world, and there is a great deal of interest in safe and effective vaccines. The efficacy of a vaccine depends not only on the antigenic component, but also on the adjuvant, which stimulates the immune system in a more effective way. Adjuvants are defined as components added to a vaccine to improve the immune response to an antigen. In addition, adjuvants have several advantages such as reducing the amount of antigen per dose of vaccine and the number of vaccinations, and in some cases they increase the stability of the antigenic component, prolonging its half-life and indirectly improving its immunogenicity. Many different types of adjuvants are available for vaccine production (Table 1).
Table 1 Classification of adjuvants according to their main mechanism of action
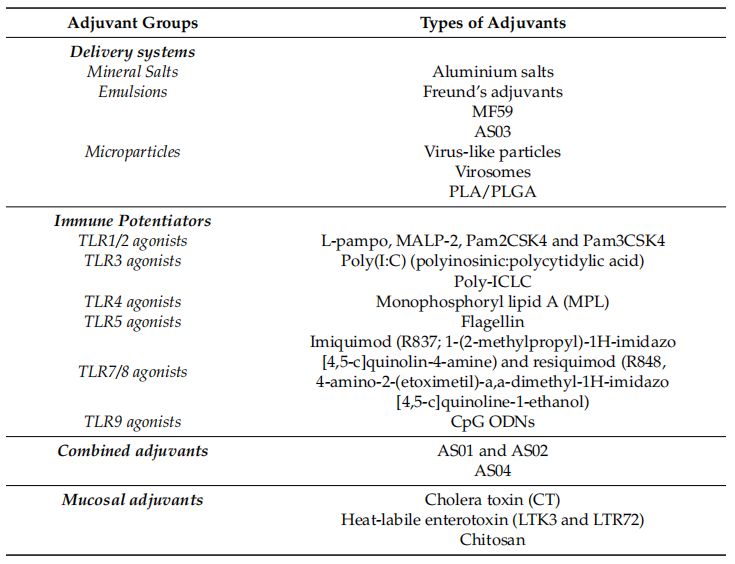
Adjuvants are grouped according to different criteria, such as their physico-chemical properties, origin and mechanism of action. One of the most popular classification systems is based on their mechanism of action, which is divided into two main groups: delivery systems (particles) and immune enhancers. The other class of adjuvants is the mucosal adjuvants, a group of compounds that share certain characteristics with the previous adjuvants. In delivery system adjuvants, the antigen is combined with the adjuvant, especially as an antigen carrier. In addition, they are capable of inducing a local pro-inflammatory response by activating the innate immune system, leading to the recruitment of immune cells to the site of immunity. Precisely, antigen-adjuvant complexes activate the pattern recognition receptor (PRR) pathway via pathogen-associated molecular patterns (PAMPs). This causes activation of innate immune cells accompanied by the production of cytokines and chemokines. The same pathway is directly activated by immune-enhancing agents (Figure 1).
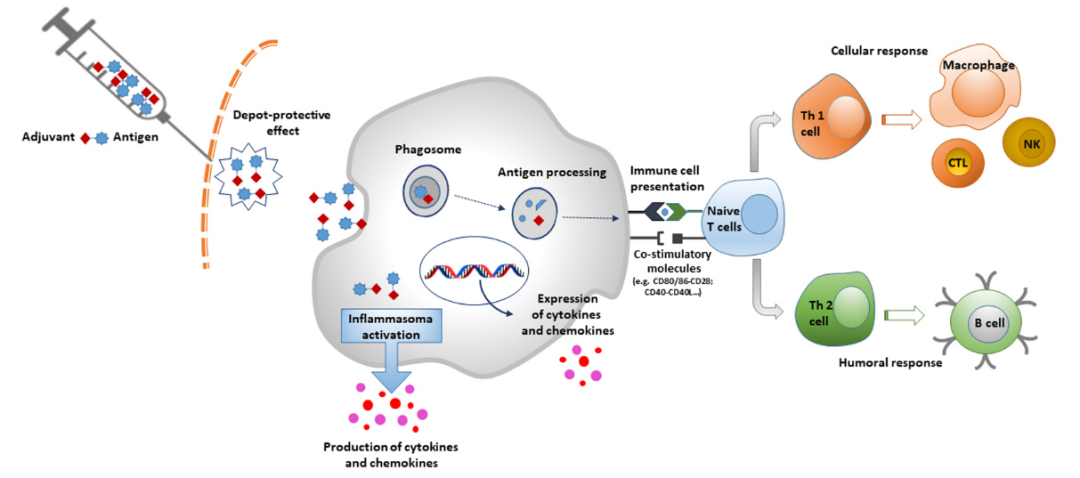
Figure 1 Mechanism of action of adjuvants
The addition of adjuvants is particularly effective for vaccines used in the elderly, since this group of subjects is subject to the physiological phenomenon of immune senescence, which leads to a weakening of the immune response after a natural infection or an artificial stimulus (vaccination). In this case, the presence of an adjuvant allows the vaccine to overcome this limitation. Moreover, adjuvants are particularly useful for subunit vaccines, which are usually weakly immunogenic and unable to stimulate a strong immune response on their own. However, not all vaccines require adjuvants. For example, the certified meningococcal conjugate vaccines do not contain adjuvants because binding to the protein carrier by itself stimulates a good immune response. Table 2 lists currently certified adjuvanted vaccines.
Table 2 Current FDA- and EMA-approved human adjuvanted vaccines
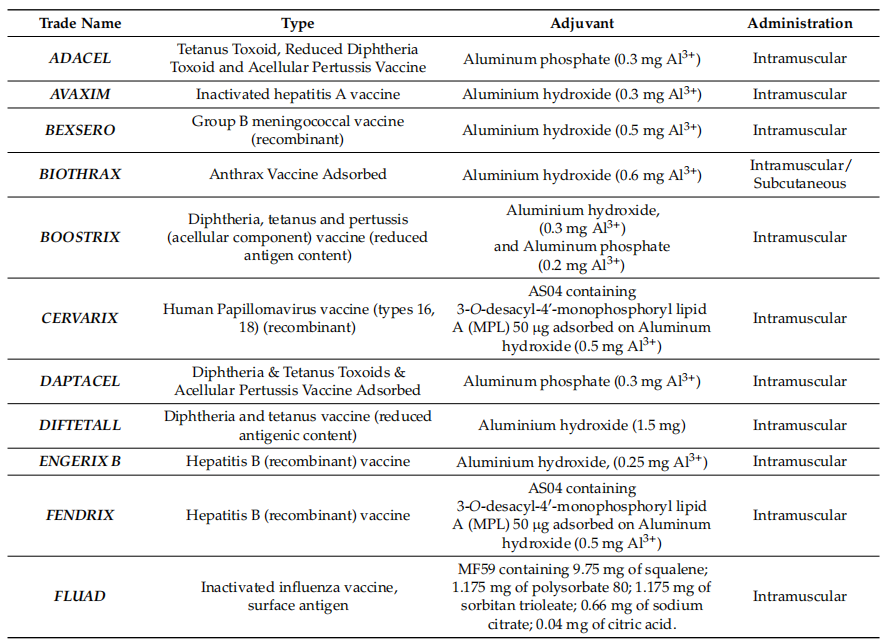
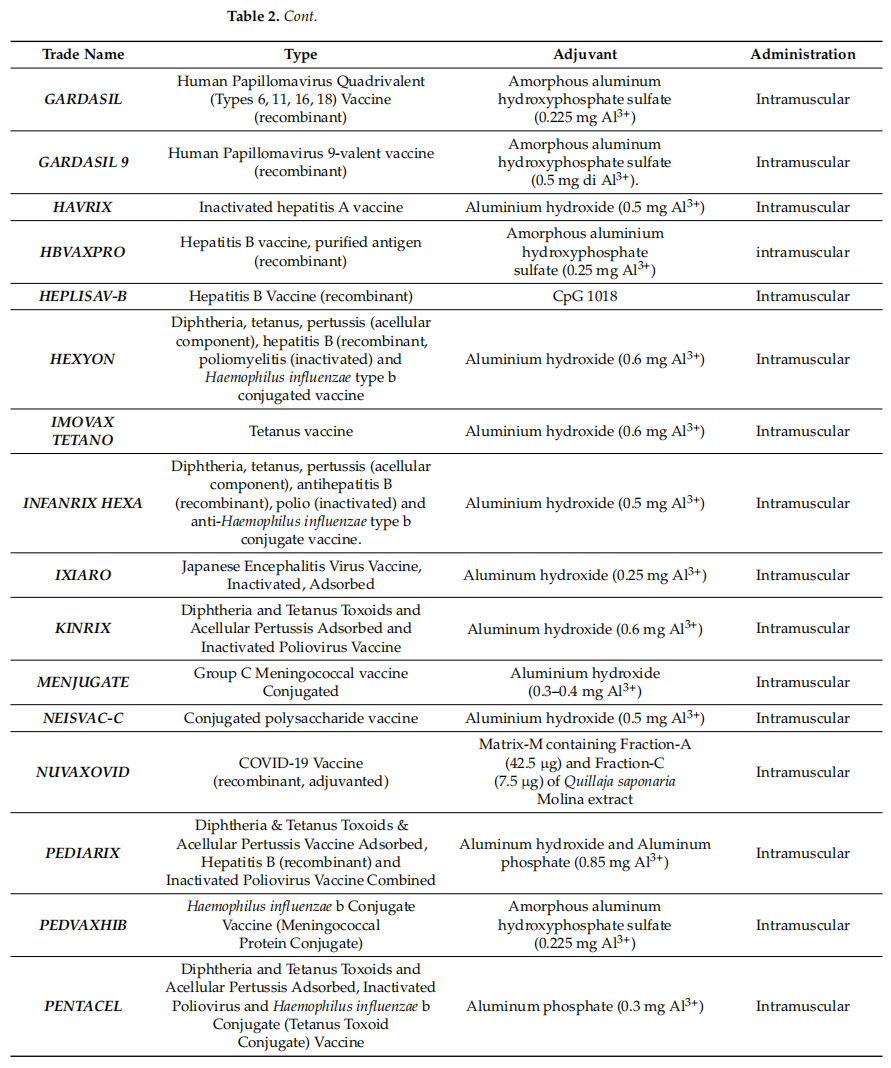
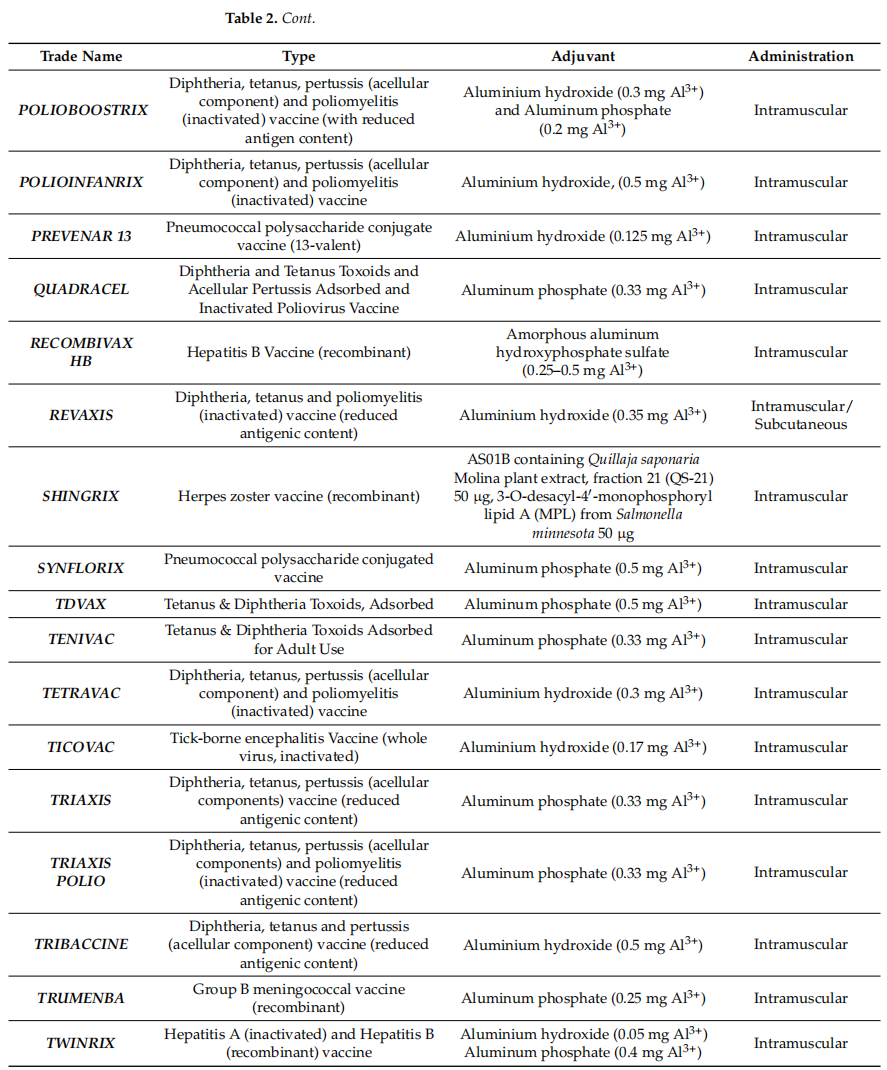
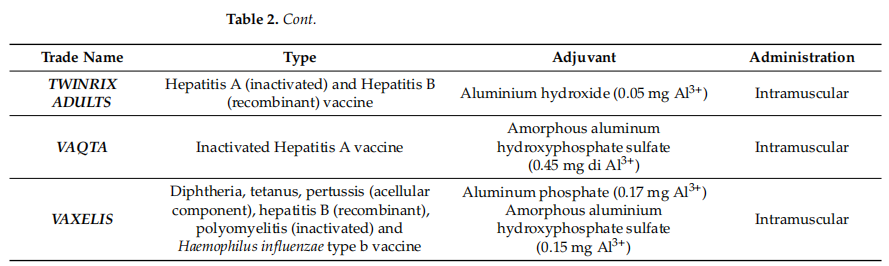
As shown in the table, the vast majority of human vaccines currently approved by the EMA and FDA include aluminum salt adjuvants. Given that these adjuvants are the earliest adjuvants used in vaccine formulations, and that an increase in the number of new adjuvants is absolutely necessary in order to improve the safety and efficacy of vaccines, there is a need to intensify research on new molecules and factors with adjuvant properties and to increase the number of in vitro and in vivo studies. At the same time, new product approvals may be subject to delays and increased costs due to regulatory challenges in the use and study of adjuvants, the use of new cellular substrates, or process change or transfer applications. This aspect may act as a barrier to discourage novelty, increase costs and delay vaccine applicability, especially in resource-poor countries.
Furthermore, the 2019 coronavirus disease pandemic highlights the importance of obtaining an effective vaccine to address the potential threat of a new pandemic. Indeed, the RNA vaccine for COVID-19 has intrinsic adjuvant properties, which are associated with the liposome component of the encoded RNA vector. However, the latest certified COVID-19 vaccine is based on a classical platform containing the spicule protein with the addition of a new adjuvant called Matrix-M, which contains components A and C of saponin extract.
There are many aspects to consider when choosing a vaccine adjuvant, of which safety comes first. A good adjuvant must be safe, well tolerated and easy to produce; have good pharmacological properties (pH, osmolality, endotoxin levels, etc.) as well as a long shelf life; and finally, be economically viable. Balancing all these characteristics without compromising the safety of the adjuvant is difficult. As a result, there are fewer adjuvants used in vaccines currently in use.
Despite the great achievements of vaccines, many concerns have arisen about these products in recent decades. A culture of opposition to vaccines, known as "vaccine hesitancy", has spread around the world. 2019 coronavirus disease (COVID-19) pandemic stimulated "vaccine hesitancy", which on the one hand demonstrates the importance of vaccination as a fundamental weapon against infectious diseases, but on the other hand highlights the hesitant behavior of some people. On the one hand, it demonstrates the importance of vaccination as an essential weapon against infectious diseases, but on the other hand, it also highlights the hesitant behavior of some people. Several reasons for this attitude have been reported in many studies, but many studies have shown that the most important reasons are the fear of vaccine side effects and mistrust of the vaccine composition, especially since adjuvant is an ingredient that attracts more public attention than the others. In fact, vaccine side effects are usually mild and transient, and they usually manifest themselves in the form of localized pain and erythema at the site of injection, mild general malaise and flu-like symptoms; these effects usually disappear after a few hours or days of vaccination, and there are few case reports of allergic reactions or other serious side effects.
This review evaluates currently used vaccine adjuvants, assesses ongoing research on adjuvant characterization and possible future use, and highlights potential problems and the basis for side effects in the scientific literature.
2.1 mineral salt
aluminum salt
The adjuvant properties of aluminum salts were discovered in the 1920s, and in 1926 people began using these compounds as vaccine adjuvants. Initially, the addition of aluminum salts to the culture medium was considered in order to induce the precipitation of tetanus and diphtheria antigens and thus aid in their purification. However, the results showed that aluminum-precipitated antigens were more immunogenic than soluble antigens. As a result, aluminum salts are the longest and most commonly used adjuvants, with approximately one-third of currently certified vaccines containing aluminum. As a result, aluminum salts are the most safety-tested adjuvants in vaccine adjuvants.
Humans are exposed to aluminum in different ways, especially through food and air. It is mainly absorbed by the body through the digestive and respiratory tracts, followed by diffusion and then a three-step elimination process, which is never completed. Less than 3% of inhaled aluminum and 1% of ingested aluminum diffuse throughout the organism. However, 95% of the aluminum found in the human body is ingested through contaminated food. The World Health Organization (WHO) has set the maximum level of aluminum intake through food at 1 mg/kg/d (60 to 70 mg/d for adults). Finally, aluminum is contained in parenteral solutions and, therefore, diffuses by injection into the blood and various body fluid compartments, in which case its concentration in intravenous solutions should be <25 g/L in order to avoid aluminum accumulation.
Once absorbed by an organism, aluminum diffuses through the body's tissues. Most of the metal is stored in the bones, liver, lungs and nervous system. In people with chronic kidney disease, aluminum cannot be removed and accumulates over time, especially in the bones and nervous system. High levels of aluminum in such patients can be deposited in the brain causing encephalopathy. The penetration of aluminum into the brain was precisely quantified in rats injected with the isotope aluminum-26 (²⁶Al). Under physiological conditions, the brain penetration of aluminum was quantified as 0.001% to 0.005% per gram of brain tissue, independent of the route of administration and chemical form.2010 Goullé et al. quantified aluminum levels in human tissue by applying inductively-coupled plasma coupled with mass spectrometry to 20 deceased patients who had no previous exposure to the metal and who had not received any treatment containing aluminum or other trace minerals, the median results, expressed as wet weight, were as follows: lung = 0.47 g/g, brain = 0.19 g/g, liver = 0.15 g/g, heart = 0.10 g/g, muscle = 0.08 g/g and kidney = 0.06 g/g.
Aluminum is slowly excreted by organisms primarily through the urinary system. Some aluminum is permanently deposited in the body, and the amount increases with exposure and age. The amount of aluminum permanently deposited in the adult body is about 30 to 50 mg.
Aluminum in vaccines exists as crystalline aluminum hydroxide (AlH) complex polymers or as clustered nanoparticles of amorphous aluminum hydroxyphosphate (AlP).AlH appears as acicular nanoparticles (ø20 nm), whereas AlP appears as a reticulated form under transmission electron microscopy. Both forms are usually soluble in citrate, but AlP is more soluble than AlH. The antigen adsorbs to the surface of the adjuvant particles by electrostatic interactions and ligand exchange. Aluminum salt antigen binding enhances antigen uptake and presentation by antigen-presenting cells (APCs). Moreover, aluminum salts stimulate the action of NLRP3 inflammatory vesicles, leading to the production of IL-1β and IL-18, causing local inflammation and recruitment of APCs.
Many vaccines are antigenically adsorbed onto AlH or AlP (e.g., diphtheria and tetanus vaccines, acellular pertussis vaccine, hepatitis B vaccine, and pneumococcal and meningococcal vaccines). Because they are weakly immunogenic, they need to boost the immune response in order to elicit an effective vaccination. In Europe, the European Pharmacopoeia specifies a maximum level of aluminum in vaccines of 1.25 mg/dose. In the United States, the Code of Federal Regulations (CFR) sets the level of aluminum in biological products (including vaccines) at 0.85 mg/dose. Aluminum is found primarily in foods as soluble citrate or chloride salts, whereas adjuvants of inorganic aluminum compounds are insoluble as part of the mode of action of the adjuvant. Therefore, absorption of aluminum from the vaccine after intramuscular or subcutaneous injection is expected to be very slow due to its poor solubility at physiological pH.
To assess the kinetics of aluminum after intramuscular injection, Flarend et al. performed some in vivo studies by intramuscular injection of 26Al-labeled AlH and AlP at a total aluminum dose of 0.85 mg, which resulted in an absorption of 17% for AlH and 51% for AlP over the 28 days of the experiment.26The maximal serum concentration of 26Al (Cmax) was 2 µg/L, i.e., the normal value for aluminum in rabbits ( 30µg/L) is 7%. Based on these results, the expected increase in aluminum Cmax after intramuscular injection of an aluminum salt vaccine adjuvant is 0.04 µg/L, i.e., 0.8% of the mean blood aluminum level of 5 µg/L. In this trial, the concentration of aluminum levels in the brain ranged from 10 - 8 to 10 - 7 mg/g, i.e., 10-5 to 10-4 µg/g, and thus <0.0001 µg/g, which is more than 2,000 times lower than the average concentration of 0.2 µg/g in the human brain. In addition, several studies have evaluated the excretion of aluminum in the human body following injection of 26Al citrate. A rather old study showed that after intravenous injection of 26Al citrate, 59% of the injected dose was excreted in the urine within one day, with slower excretion in the following days (average retention rate of 27% on day 5), while a more recent study showed a residual rate of about 2% after 8 years of aluminum injection.
The toxicity of aluminum is second only to the increased levels of the metal in body fluids and tissues. Especially when renal function is impaired, this increase is altered by the ability to eliminate it. Patients with renal failure on hemodialysis in particular have high aluminum levels accompanied by a possible risk of neurotoxicity. Kidney transplantation can address the aluminum excess and possibly the associated neurotoxicity. For many years, scientists have debated that neurodegenerative diseases may be neurotoxic due to aluminum, and to date, there is no conclusive evidence of this role, which remains controversial.
The neurotoxicity of aluminum has been studied in vitro, in isolated and in vivo animal models and in humans. A number of in vitro studies in bacteria have shown that aluminum is not mutagenic and, in addition, questions have arisen about the mode of oxidative action of aluminum following the use of strains sensitive to oxidative mutagens. Cell lines were studied in vitro to assess the possible genotoxicity of different forms of aluminum. Researchers conducted in cell lines to assess the possible genotoxicity caused by different forms of aluminum. Some scientists have conducted embryotoxicity studies in animal models using lymphocytes from multiple donors and, finally, various studies in vivo. The most commonly used techniques in these studies are the comet assay and the micronucleus test. Interestingly, the results of the studies are often inconsistent and contradictory, which may have methodological flaws. Thus, to date, it cannot be said with certainty (at recommended doses) that aluminum salts used as adjuvants have toxic effects, despite their ability to produce more or less intense oxidative stress.
2.2 Emulsions
The predecessors of this important class of adjuvants were complete and incomplete Fuchs' adjuvants. Both adjuvants are water-in-oil emulsions that present antigen and stimulate the innate immune system. In its construction complete Freund's adjuvant (CFA) consists of heat-killed Mycobacterium bovis, which enhances the stimulation of the immune response and currently induces strong immune activation and autoimmunity in mice (e.g. uveitis and experimental autoimmune encephalomyelitis). However, CFA is capable of inducing intense and long-lasting localized inflammation, which may lead to significant pain in animals and possibly ulceration at the injection site. Incomplete Freund's adjuvant (IFA), which is free of Mycobacterium, was used as an adjuvant for human influenza vaccines in the 1950s and induces a stronger and longer-lasting antibody response than the same vaccine without adjuvant.The adjuvant activity of IFA is based on the characteristics of oily antigen deposition, with a sustained release of the antigen at the site of injection, which results in both an increase in the lifespan of the antigen as well as a strong localized innate immune stimulus accompanied by phagocytosis, leukocyte recruitment and infiltration, and cytokine production. However, the application of IFAs in vaccine formulations and their routine use in humans is hampered by the strong side effects of IFAs. In particular, the toxicity is caused by the high content of non-biodegradable used oils and their poor quality.A survey conducted by WHO in 2005 showed that about 1 million IFA patients immunized developed serious side effects, such as sterile abscesses in 40,000 vaccinated individuals.
2.2.1 MF59
MF59 is a water-in-oil emulsion consisting of squalene, Span 85 and Tween 80 dissolved in 10 mM sodium citrate buffer at pH 6.5, with an average particle size of approximately 165 nm.This was the first oil-in-water emulsion approved for use as a human vaccine in Italy in 1997. It is currently used in the trivalent and quadrivalent (TIV and QIV) influenza vaccines Fluad (Seqirus), which were initially used only in people over 65 years of age but were later approved for other influenza risk groups such as toddlers and infants, and for pregnant women and young children during the H1N1 pandemic vaccine. Studies have shown that the presence of MF59 improves the effectiveness of influenza vaccines in children under 2 years of age.MF59 has also been tested as an adjuvant for HBV vaccines, where it elicits a strong immune response and is better than that induced by aluminum.The mechanism of action of MF59 is similar to that of aluminum salts. The storage status at the injection site is almost negligible, as studies have shown a half-life of 42 h. In contrast, MF59 has a potent ability to induce cellular and humoral immune responses, including the production of high titers of functional antibodies.The presence of MF59 stimulates the secretion of chemokines by local innate immune cells, such as C-C motif chemokine ligand 4 (CCL4), C-C motif chemokine ligand 25 (CCL2), C-C motif chemokine ligand 5 (CCL5), and C-X-C Motif ligand 8 (CXCL8), which in turn drives leukocyte recruitment, antigen uptake, and migration to lymph nodes, triggering adaptive immune responses. Additionally, studies have reported that MF59 increases the expression of a cluster of genes that regulate leukocyte migration across the endothelium and the subsequent recruitment of MHCII+CD11b+ cells into the injection site, triggering a robust immune response. Millions of doses in 35 countries have demonstrated that MF59 is safe and well tolerated.
2.2.2 AS03
AS03 is a water-in-oil adjuvant emulsion consisting of the surfactant polysorbate 80 and two biodegradable oils, squalene and DL-alpha-tocopherol, in phosphate-buffered saline. This adjuvant has been used in influenza vaccines, eliciting an immune response similar to that of MF59, and in malaria vaccines.In 2009, the European Commission approved the marketing of the AS03-adjuvanted vaccine, Pandemrix, and in 2013, the U.S. Food and Drug Administration (FDA) was granted approval for an AS03-adjuvanted influenza A (H5N1) monovalent vaccine. However, the antioxidant and immunostimulatory properties of alpha-tocopherol appear to enhance immunostimulation over MF59. Indeed, the use of AS03-adjuvanted influenza vaccine in children aged 6 to 35 months showed a strong immune response, even 6 months after vaccination. To elucidate the role of DL-α-tocopherol in AS03, we compared the effects of AS03 with those of an emulsion lacking DL-α-tocopherol. By measuring the levels of antigen uptake, immune cell recruitment, and secreted cytokines, we concluded that the lack of DL-α-tocopherol resulted in reduced immune response and antibody titers. Moreover, it has been shown that AS03 is able to stimulate the immune system by activating NF-κB, inducing cytokine and chemokine secretion from muscles and lymph nodes, and promoting the migration of innate immune cells. In addition, AS03 stimulates CD4+ T cell-specific immune responses, which can determine long-lasting neutralizing antibody production and higher levels of memory B cells.The composition of AS03 is further enhanced with two strong immunostimulants, QS-21 (a saponin extracted from Astragalus membranaceus) and 3-O -desmethyl-40 -monophosphoryl lipid A (MPL), to enhance its immunogenicity, resulting in the formation of AS02.
2.3 Particulate
2.3.1 virus-like particle
Virus-like particles (VLPs) are icosahedral or rod-shaped nanoparticles (Ø 20 - 200 nm) composed of self-assembled capsid protein shells. They have long been studied and used for vaccine development. They are non-infectious particles as they do not contain any genetic material. They are one of the most important representatives of a new type of vaccine called nanovaccines, which are becoming more and more important in vaccine development.VLP is a smart nanoparticle because they are external viral capsid formations with repetitive epitopes, which are recognized by the immune system as not being self, resulting in a strong immune response. However, it shares this property with natural viruses but does not have the ability to infect. In addition to these repetitive structural motifs, VLPs are similar in size to viruses (typically between 20-800 nm) and are processed quickly and efficiently to produce a rapid and long-lasting immune response even in the absence of adjuvants. Depending on the presence or absence of an envelope, VLPs can be categorized as non-enveloped VLPs and enveloped VLPs (eVLP), and non-enveloped VLPs can be further categorized as single-coated and multi-coated protein VLPs, as well as mono-, bi-, and trilamellar VLPs. multi-coated, non-enveloped VLPs A classical example of multi-enveloped VLPs is formed by papillomavirus L1 and L2 proteins, which are capable of self-assembling to form particles. eVLPs acquire lipid membranes from host cells, are expressed during assembly and outgrowth, and are subdivided into monolayers, bilayers, and multilayers. They can be formed from different viral types and their expression systems include E. coli, yeast (Saccharomyces cerevisiae and Picrosporum), baculoviruses, mammalian cells, plant cells and cell-free systems. Production of VLP in cellular systems uses a multi-step approach called "assembly-then-purification", which first takes advantage of the spontaneous assembly ability of coat proteins to appear directly in the expression cell vector, and second purifies the newly formed particles. Sometimes, in order to obtain purer particles, the new particles must be disassembled after intracellular assembly so that they can be recombined a second time. Another manufacturing method uses cell-free in vitro assembly processing systems with a composition opposite to that of traditional cell-based methods; in particular, after they are expressed and purified, the in vitro expression system is used as a platform for the production of spontaneous assembly of coat proteins without the need to disassemble the newly formed VLPs.Currently, two important adjuvant-added vaccines use nanoparticle platforms to generate immunity: the hepatitis B and the papillomavirus (HPV) Vaccines. Currently, the hepatitis B vaccine in use is a recombinant vaccine containing hepatitis B surface antigen (HBsAg) in the form of VLPs for the prevention of hepatitis B infection, which utilizes recombinant DNA technology to produce the vaccine using Saccharomyces cerevisiae as an expression vector. Each dose contains 10 µg/0.5 mL of VLPs (children) or 20 µg/mL (adults), both adsorbed on aluminum hydroxide. The vaccine is administered to infants, children, and adolescents under 15 years of age, or those at high risk for hepatitis B infection, and has also shown excellent immunogenicity (95% - 99% efficacy) in newborns born to mothers with hepatitis B virus. The hepatitis B vaccine appears to provide immunity for at least 10 years.
HPV vaccines are also based on the VLP platform.HPV viral particles are non-enveloped and contain double-stranded DNA (dsDNA). The capsid has icosahedral symmetry and consists of a major structural protein and a minor structural protein, the L1 protein and the L2 protein. The current nine-valent HPV vaccine protects against nine different viral genotypes that cause 90% of cervical cancers and 80-95% of anogenital cancers, and is recommended for both males and females beginning at age 9. The nine-valent HPV vaccine contains L1 proteins of nine different HPV genotypes (6, 11, 16, 18, 31, 45, 53, 58) to form VLPs, which are synthesized by recombinant DNA technology.The advantage of VLPs is that it is a protein structure without viral genome, which is not infectious or carcinogenic. The vector currently used to express the L1 protein is Saccharomyces cerevisiae.VLPs used in synergy with adjuvants (AlP) produce a favorable immune response and therefore prevent cervical cancer by up to 90%. In addition, vaccine-induced antibodies have been shown to cross the placenta and protect newborns against HPV 6 and 11.
2.3.2 virus particle
Virosomes are a vaccine platform that closely resembles the structure of natural viruses. Structurally, they are recombinant influenza virus envelope-forming VLPs composed of hemagglutinin (HA), neuraminidase (NA), and phospholipids (phosphatidylethanolamine and phosphatidylcholine) that lack viral genetic material.The use of viral particles in the production of influenza vaccines was first proposed in 1975. Since then, scientific evidence of the efficacy of such vaccines has emerged and two vaccines have been used for the prevention of hepatitis A (Epaxal) and influenza (Inflexal).Inflexal V is an adjuvanted influenza vaccine for all age groups, with favorable efficacy in healthy and immunocompromised children, adults, and the elderly. It induces a B-cell response and produces specific antibodies. The viral particles retain the receptor-binding capacity and membrane fusion activity of hemagglutinin, but lack viral RNA; they bind and do not induce cellular infection. Moreover, this binding capacity increases their immunogenicity compared to subunit and split virus particle influenza vaccines. Viral particles act as a perfect delivery system, capable of transferring antigens into the cytoplasm of antigen-presenting cells (APCs) and inducing cytotoxic T-lymphocyte (CTL) responses. However, due to their weaker adjuvant properties, viral particles are not effective in activating APCs and promoting cross-presentation. This intrinsic limitation can be removed by adding stronger adjuvants. For example, a novel influenza vaccine was developed based on viral particles adsorbed to membranes with the TLR4 ligand monophosphoryl lipid A (MPLA) and the metal ion chelating lipid DOGS-NTA-Ni. In vitro, virus particles adsorbed with MPLA induced stronger activation of APCs compared to virus particles without added adjuvant. In addition, virus particles with MPLA adjuvant immunized mice induced the production of specific CTLs.
Influenza virus particles were produced using detergent (ethylene glycol)-n-dodecyl monoether (C12E8) to solubilize the viral envelope, followed by ultracentrifugation and removal of the viral nucleocapsid, and then, using hydrophobic beads to remove the detergent from the supernatant, followed by the reassembly of the viral membrane lipids and the envelope glycoproteins, to form particles of approximately 100-200 nm. It was shown that influenza virus particles formed by this process exhibit fusion properties very similar to those of wild-type virus. Influenza viruses enter cells through receptor-mediated endocytosis and then fuse with the nuclear endosomal membrane. Due to the membrane fusion activity, different macromolecules can be encapsulated within the lumen of the viral particle to reach the cytoplasm of the target cell. For example, DTA (A subunit of diphtheria toxin) encapsulated within the viral particle can be successfully translocated into the cytoplasm of the target cell, leading to complete inhibition of protein synthesis, and even plasmid DNA can be encapsulated within the cationic lipid-forming viral particle. This viral particle DNA can efficiently transfect target cells.
Viral particle delivery systems and adjuvants have the significant advantage of being able to adsorb antigens to their surfaces and lumens through hydrophobic lipid interactions. Moreover, virus particles are preferred over VLPs in vaccine production because the latter are limited due to their protein structural motility. In addition, adsorption of antigens onto the surface of the liquid phospholipid bilayer of viral particles stimulates interactions with host cell receptors.The FDA has approved viral particles as nanocarriers for human use because of their high tolerability and safety. In contrast to subunit vaccines, which elicit weaker immune responses, viral particles induce strong humoral and cellular immunity in a very similar manner to natural infections and other potent adjuvants.
So far, in addition to the two viral particle-based vaccines for influenza and hepatitis A mentioned above, these several viral particle-based vaccines for HIV, HPV, RSV, and malaria are also under study.
The HIV particulate vaccine has shown results consistent with expectations in clinical phase I and is likely to be available soon. Although the vaccine can be administered intramuscularly or subcutaneously, the mucosal route is likely to elicit a stronger immune response because the primary route of HIV transmission is through mucosal tissues, and therefore, robust mucosal antibody production is an important defense mechanism against HIV infection. Researchers have prepared HIV virus particle-based vaccines from influenza viruses by adsorbing a number of HIV-1 virulence antigens, such as gp41 and p1 peptides, with 3M -052 and a heat-resistant adjuvant that increases the membrane hardness of the virus particles. In another study, a heat-stable HIV-1 virus particle vaccine consisted of influenza-enveloped virus particles containing HA, NA, lecithin, ceruloplasmin, and other phospholipids, plus 3M-052, toll-like receptors (TLR7/8), and alginate.
With respect to HPV, several studies have focused on viral particle-based vaccines containing E6 and E7 proteins that fuse with host cell membranes via receptor-mediated endocytosis. It was shown that recombinant HPV16 E7 influenza virus particles induced a strong CTL response and blocked HPV16+ transformation to cancer. In addition, immunization with E7 virus particles induced an IgG antibody response against E7.
3.1 TLR1/2 agonist
Among the TLR1/2 agonists, L-pampo is a potent adjuvant system consisting of Pam3Csk4 (Pam3) and polyinosinic:polycytidylic acid (polyI:C), which are potent TLR1/2 and TLR3 agonists, respectively.Lee et al. demonstrated that L-pampo induced stronger anti-HBV antibody production than alum and was also involved in cell-mediated immune responses, such as increased multifunctional CD4+ T cells. In addition, effective adjuvants against SARS-CoV-2 have been investigated. Specifically, SARS-CoV-2 antigens such as receptor-binding domain (RBD) and S1 antigens, or RBD- Fc splicing L-pampo produced strong humoral and cellular immune responses against SARS-CoV-2 compared to widely used adjuvants.
In addition, bacterial lipoproteins are the most efficient ligands recognized by TLR2. Studies have shown that synthetic lipopeptides derived from bacterial lipoproteins are strong activators of B cells and macrophages and can be used as vaccine adjuvants. The 2 kDa macrophage-activating lipopeptide-2 (MALP-2) from Mycoplasma fermentans activates immune cells through TLR2- and myd88-dependent signaling pathways. In addition to MALP-2, Pam2CSK4 and Pam3CSK4 are recognized TLR2 agonists that have been evaluated for the treatment of infectious diseases such as Leishmania protozoa, malaria and influenza.
TLR3 receptor agonist
Prior to the discovery of TLRs, a synthetic dsRNA, [poly(I:C)], was found to be highly capable of inducing IFN production.TLR3, a receptor that detects viral dsRNAs in the endosomes of the cell nucleus, recognizes poly(I:C) because it structurally mimics the viral RNAs, thereby inducing the production of both type I IFNs and type III IFNs and causing a Th1 cytokine response. Type I IFN production following TLR3- poly(I:C) interaction is particularly important for efficient activation of CD8 T cell responses by conventional dendritic cells (cDCs). In addition, poly(I:C)-generated type I IFN stimulates clonal expansion of T cells, increasing the proportion of effector T cells and the number of antigen-specific B cells. For these reasons, poly(I:C) has been extensively studied as a potential adjuvant; however, poly(I:C) has toxic effects on humans. Therefore, the attention of scientists has been focused on poly(I:C) derivatives, such as poly(ICLC) and poly(IC12U), as well as other synthetic TLR3 agonists, such as ARNAX, IPH3102, and RGC100. poly(ICLC) is poly-L-lysine in carboxymethylcellulose, and, similar to poly(I:C) Similarly, it stimulates IFN production. However, it is highly resistant to serum nuclease and also has a high immunostimulatory effect. an interesting aspect of poly(ICLC) is the ability to induce the expression of several other gene sequences in the natural immune pathway, including the inflammasome and complement system AS, similar to live virus vaccines. To date, several studies have used poly(ICLC) as a vaccine candidate for infectious diseases such as Plasmodium falciparum and HIV as well as for cancer. It has been shown that poly(ICLC) triggers a stronger Th1 immune response than other TLR agonists (e.g., LPS and CpG), which is a positive aspect of vaccination. poly(IC12U) reduces the toxicity of poly(I:C) by mismatching between uracil and guanosine residues. However, although this change reduces toxicity, it results in a lower yield of type I IFN than poly(I:C). Unlike poly(I:C) and poly(ICLC), poly(IC12U) binds to TLR3 but not to MDA5. Similar to poly(ICLC), some studies have used poly(IC12U) as an adjuvant for HIV, influenza, and cancer vaccines. arnax, a TLR3 agonist with adjuvant potential, is a TLR3-specific ligand with lower toxicity than poly(I:C). poly(I:C) is less toxic than poly(I:C) with its activation of the MAVS pathway (RIG-I and/or MDA5 activation) is related to its ability to activate the MAVS pathway (RIG-I and/or MDA5 activation). Therefore, Matsumoto et al. developed a ligand comprising a GpC-phosphorylated oligodeoxynucleotide and dsRNA that is recognized by TLR3 and internalized into the nuclear endosome. Due to the relatively short length of the RNA strand, this ligand was able to activate TLR3 while avoiding detection of MDA5. In a mouse model, the adjuvant failed to induce a significant increase in serum inflammatory cytokine levels, but favored DC cross-presentation of antigen and triggered Th1 profiling. Two of the most important areas for studying ARNAX are cancer immunotherapy and influenza vaccination.
3.2 TLR4 agonist
TLR4 agonists used as vaccine adjuvants are AS01, AS02 and AS04, all of which contain the ligand MPLA for TLR4 endosomes.
Specifically, AS01 has been used in the development of vaccines for malaria, HIV, and tuberculosis.AS01 is a composite adjuvant encapsulated in a liposomal structure that consists of two different immune-stimulating molecules, MPLA and QS-21, a naturally occurring triterpene glycoside saponin analog derived from the bark of the loquat paste. Both compounds utilize liposomes as carriers to reach cells via cholesterol-dependent endocytosis. Intracellularly, QS-21 causes lysosomal destabilization and promotes activation of the protein kinase SYK. MPLA connects to the endosomal TLR4 and induces a TRIF-dependent signaling pathway. QS-21 alone has an important and unfavorable hemolytic effect and induces cell death. However, hemolytic activity of QS-21 and subsequent cell death is terminated by liposome encapsulation.AS01 activates apoptotic protease-1, which promotes the activation of NLRP3 inflammasomes and the release of IL-1β and IL-18 from APCs.The release of IL-18 leads to the rapid production of IFN-γ, especially in natural killer cells in lymph nodes, which promotes maturation of DCs and induction of Th1-type immune responses.
3.3 TLR5 agonist
TLR5 is a receptor that recognizes bacterial flagellin, which is expressed by several immune cells.Binding of TLR5 to ligands results in activation of inflammatory pathways and release of many inflammatory mediators, such as TNF-α, IL-1β, IL-6, and NO.In addition, flagellin is capable of eliciting both Th1 and Th2 responses unlike the other TLR ligands, which are capable of eliciting only Th1 responses. In addition, flagellin induces IL-1β production and release by activating the NLRC4 inflammasome. Models in which flagellin is not dependent on TLR5 or NLRC4 are able to exert adjuvant activity, but at a lower efficiency than wild type. Indeed, the ability to adjuvinate was greatly reduced in mouse models in which neither receptor was present, suggesting that at least one receptor needs to be present to drive an immune response; and the presence of both provided the best immune outcome. It has been shown that flagellin maintains its adjuvant effect in immunocompromised populations, such as HIV-positive patients.Studies using flagellin as an adjuvant were reviewed by Cui et al. The simplest method is antigen administration, which has been successful in inducing mucosal immune responses that are essential for preventing respiratory and gastrointestinal infections. Researchers have conducted many studies, particularly on the role of flagellin as an adjuvant for influenza vaccines. Salmonella typhimurium flagellin in combination with different influenza antigens such as inactivated influenza virus PR8 (IPR8), HA (H5N1), and avian influenza virus (AIV) H5N1 have yielded a robust immune response (especially IgA-producing mucosa). Chimeric flagellin or flagellin-antigen complexes were obtained for flagellin modified in live attenuated bacteria, such as Mycobacterium tuberculosis, Vibrio cholerae, Streptococcus pyogenes, Listeria monocytogenes and enterotoxin-producing Escherichia coli (ETEC). In addition, the production of recombinant flagellin-antigen fusion proteins has been used in animal models for adjuvant vaccines against infectious diseases and cancer. To date, at least three vaccines using flagella as adjuvants are in clinical trials: two for influenza virus and one for Yersinia pestis.
3.4 TLR7/8 agonists
Several studies have shown that TLR7/8 agonists strongly induce the Th1 immune response. Ligands bound to TLR7/8 produce high levels of type I IFN, IL-12, TNF-α and IL-1β. In addition, TLR7/8 and TLR9 agonists are the only agonist molecules capable of activating and promoting the clonal expansion of cDCs and plasma cell-like dendritic cells (pDCs), as well as mobilizing CD14+CD16+ inflammatory monocytes and CD14dimCD16+ patrolling monocytes.The most important representatives of TLR7/8 agonists are a number of synthetic small molecules named imiquimod (R837) and riquimod (R848), which belong to the imidazoquinoline class. Today imiquimod is approved for the treatment of genital warts, superficial basal cell carcinoma, and actinic keratosis, while resiquimod has been investigated for use in antiviral and anticancer therapy. However, these small molecules have been shown to have some inherent limitations. In particular, they can diffuse away from the site of administration and thus away from the antigen, thereby reducing efficacy and inducing systemic side effects. Therefore, studies have demonstrated that direct binding of these molecules to aluminum adjuvants can enhance vaccine efficacy. Several previous studies have directly coupled imidazoquinolines to HIV-1 Gag proteins or whole inactivated influenza viruses, increasing the number of Th1-responsive and antigen-specific T cells. In addition, its coupling to synthetic polymer scaffolds, lipid-polymer amphiphiles, polyethylene glycol (PEG), nanogels, bright aluminum, and a variety of other synthetic polymers significantly increased the delivery of imidazoquinolines and promoted the maturation of DCs and antigen-specific T cells. Moreover, studies of imidazoquinolines in combination with one or more other TLR agonists, such as MPLA (TLR4) and MPLA + CpG ODN (TLR4 and TLR9), demonstrated that such combinations increased innate immune responses, produced significant antigen-specific neutralizing antibodies and improved Th1 responses. All these innovative aspects highlight the excellent potential of TLR7/8 agonists as adjuvant candidates.
3.5. TLR9 agonist
TLR9 naturally recognizes bacterial DNA motifs represented by unmethylated cytosine-phosphate-guanine (CpG) dinucleotides that drive the activation of the innate immune system through a myd88-dependent pathway. These molecular motifs are used in synthetic adjuvants with specific modifications to prevent degradation by nucleases. In natural killer cells, B cells, and pDCs, CpG-ODNs produce potent chemokines, cytokines, and antibodies that drive strong Th1-type immune responses. To date, researchers have developed three different types of CpG-ODN ligands, which are classified into three classes (A - C), but only class B molecules have been used in clinical trials as adjuvants. cpG-B ODN localizes to the nuclear endosome and induces maturation of pDCs. In addition, CpG-B ODN can interact directly with B cells and enhance antibody production. In mouse models, CpG-B ODN was shown to produce substantial and durable antibodies as an adjuvant, superior to aluminum adjuvanted or non-adjuvanted vaccines. Recently licensed CpG 1018, an oligonucleotide with high chemical stability and adjuvant capacity to elicit a Th1-type immune response, was used as an adjuvant for the hepatitis B vaccine, Heplisav-B. CpG 1018 in Heplisav-B improves vaccine efficacy, requiring only two doses compared to conventional hepatitis B vaccines, which require three doses to produce optimal protection. To date, CpG 1018 is being used in the development of several vaccines, including vaccines against melanoma and COVID-19, another CpG ODN, CpG 7909, has shown encouraging results in clinical evaluations of HBV and malaria vaccines, and other next-generation TLR9 agonists have been developed. An effective representative is MGN1703, a small DNA molecule that includes CG motifs, but which is structurally distinct from CPG ODNs.MGN1703 is a stretch of reverse-complementary DNA with a double-stranded middle surrounded by two single-stranded loops, which includes three non-methylated CG motifs and forms a dumbbell-like structure in contrast to the linear molecule CpG ODNs.MGN1703 has been used as a adjuvant for cancer vaccines was tested and found to activate both innate and adaptive immune responses with only minor or temporary side effects.
Despite the favorable safety profile of vaccines, in recent years new concerns have arisen about possible negative effects of vaccines, and in addition to well-known side effects, a new taxonomic entity of disease has been described. The first entity proposed by Shoenfeld et al. is the adjuvant-induced autoimmune/inflammatory syndrome (ASIA). This syndrome consists of a number of immune-mediated diseases that may occur in genetically susceptible individuals following exposure to adjuvants. It is characterized primarily by the production of autoantibodies, which improve once the triggering factor is removed. The syndrome develops when some external factor such as an infectious agent or an adjuvant (i.e., dust, silica gel, aluminum salts, etc.) acts on a susceptible genetic background, which is mediated through specific HLA antigens associated with the development of autoimmune disease (ADI). In particular, the coexistence of HLA-DRB1 and PTPN22 genes in these patients has been shown to be the most common autoimmune background. According to recent scientific evidence, a number of pathological conditions such as tuberculosis, Sjögren's syndrome (SS), undifferentiated connective tissue disease (UCTD), silicone implant incompatibility syndrome (SIIS), and immune-associated adverse events (irAEs) are typical examples of ASIA background. In addition to the common adjuvants contained in vaccines, many other substances such as silicone, paraffin, hyaluronic acid, acrylamide and methacrylates have adjuvant properties.
Watad et al. categorized the ASIA criteria into primary and secondary criteria. Prior to clinical manifestations, the primary criteria included exposure to various exogenous stimuli (infections, exposure to adjuvants) and the presence of typical clinical manifestations such as myalgia, myositis, arthralgia, arthritis, chronic fatigue, sleep disturbances, demyelination, memory loss, fever, and dry mouth. Secondary criteria include the presence of autoantibodies or antibodies against adjuvants, the presence of specific HLA patterns (i.e., HLA DRB1, HLA DQB1), and the development of an autoimmune disease, i.e., multiple sclerosis or systemic sclerosis.
A number of previous studies have shown that vaccines containing aluminum salts can cause ASIA. a prime example is the HPV quadrivalent vaccine (containing aluminum salts), which has been reported to increase the risk of autoimmunity in susceptible subjects within a few weeks of vaccination, and it or the HBV vaccine. However, the results of Linneberg's study of the potential side effects of aluminum salts showed that people who received multiple subcutaneous immunotherapy with allergens combined with aluminum hydroxide and who subsequently received 100 times the amount of aluminum contained in the vaccine than in the three-dose vaccine had lower mortality rates and fewer autoimmune disorders than controls receiving conventional allergy treatments.
The efforts of scientists have turned to studying biomarkers to diagnose ASIA or predict its susceptibility (in addition to those already mentioned). For example, ACE 1 and IL-2 receptors were increased by 50% in subjects with ASIA, and vitamin D deficiency increased the incidence of ASIA (lack of immunomodulation). By analyzing 500 cases of ASIA, Watad et al. emphasized that susceptibility seems to be very relevant to ASIA. This study showed a higher rate of ASIA in female individuals, smokers, those with previous autoimmune diseases or family members with the latter. Polygenic autoimmune diseases were the most common, with UCTD and Sjögren's syndrome having the highest prevalence of 38.8% and 16.8%, respectively. Of the 54.4% of patients who tested positive for autoantibodies 48.2% were ANA positive. It is clear that there are environmental and genetic factors behind the development of autoimmune/autoinflammatory states, considering their presence. The median time between vaccination and onset of symptoms was one week (2 days to 5 years); 48.2% of the population developed clinical symptoms after exposure to at least one vaccine. However, in addition to this scientific evidence, several studies have shown no relationship between adjuvanted vaccination and ASIA. In these studies, it has been shown that the link between vaccination and autoimmunity may be spurious because of confounding factors and the result of random events rather than a true causal relationship. The possible link between vaccination or exposure to foreign substances and the potential occurrence of autoimmune/inflammatory and immune-mediated events should not be used as a "false myth" to reduce vaccination coverage. In reality, adverse vaccine events are rare. In the absence of information and reliable data, Asia is an appropriate umbrella term to group together events and apparently unrelated reactions that have a common origin in exposure to vaccines, silica gel or other foreign substances. It is important to emphasize that even if future research shows a real correlation between adjuvants and autoimmunity, however, this will not diminish the enormous and unquestionable protective role played by the practice of vaccine immunization, which provides many clinical benefits; in fact, vaccines contribute to the eradication and control of many infectious diseases and to the improvement of the quality of human life.
Vaccination has always been one of mankind's most powerful weapons in the fight against infectious diseases. Thanks to these effective and safe means of prevention, mankind has been able to eliminate the worst enemies (pathogenic microorganisms) in human history. The recent COVID-19 pandemic has amply emphasized the importance of vaccination and the need for further progress in vaccine research and preparation for future pandemics. The efficacy of a vaccine depends on the essential properties and action of the adjuvant. With regard to the future of vaccination, greater emphasis must be placed on these molecules in order to produce increasingly safe and effective vaccines. Research in this area, including on related products, is currently under way in order to achieve this goal for the benefit of humankind.






