Over the past decade, monoclonal antibodies (mAb) have gained tremendous therapeutic application as efficient, flexible tools for treating a wide range of diseases. Despite this success, there is still an opportunity to reduce the production costs of antibody-based therapies by optimizing cost-effective measures. In order to reduce production costs, novel process intensification methods based on state-of-the-art replenishment batching and perfusion have been implemented over the past few years. On the basis of process intensification, we present here the feasibility and benefits of a novel, innovative hybrid process that combines the robustness of replenishment batch operation with the benefits of complete media replacement via fluidized bed centrifuges (FBC). In an initial small-scale FBC simulation screen, we investigated multiple process parameters that resulted in increased cell proliferation and prolonged activity trends. Subsequently, the highest yielding process protocol was transferred to a 5 L scale, further optimized and compared to a standard replenishment batch process. Our data show that the novel hybrid process significantly increased peak cell density (163%) and significantly increased mAb volume by ~254% while using the same reactor size and process duration as the standard recharge batch operation. Furthermore, our data show comparable critical quality attributes (CQA) between processes and reveal the potential for scale-up without the need for extensive additional process monitoring. Thus, this new process enhancement strategy has great potential for transfer to future industrial production environments.
Synopsis
Recombinantly expressed biotherapeutics, such as monoclonal antibodies (mAb), hormones, cytokines and vaccines, have received significant attention in the pharmaceutical industry in recent years. In particular, since their first clinical approval in 1986 (Orthoclone OKT3®), novel mAb-based therapeutics have become a major point of interest for clinical applications and bioproduction. Since then, numerous clinical trials have highlighted the unique advantages of these biotherapeutics, such as flexibility in adapting their application, low toxicity, high specificity and suitable in vivo half-life. As a result, as of today, more than 100 mAb-based therapeutics have been approved by the U.S. Food and Drug Administration (FDA), and many more are in clinical development, proving that interest in these biologics continues to grow.
Therapeutic antibodies are characterized by complex post-translational modifications to ensure biological activity and low toxicity of the protein. Therefore, mammalian cell lines, such as Chinese hamster ovary (CHO) cells, are used to express these complex biomolecules rather than microbial hosts. Antibody-based immunotherapies are quite costly to produce compared to small molecule therapies, resulting in them being very expensive. To reduce the cost, novel and innovative reinforcement strategies are necessary.
Discontinuous process forms such as replenishment batch (FB) are the state-of-the-art for large-scale production of mAb due to their robustness, simplicity and reproducibility. The process can be divided into three successive culture phases: a logarithmic phase of exponential cell growth, a stable phase of no cell growth, and a dead phase of decreased cell activity usually due to an increased number of apoptotic cells. In FB processes that prevent nutrient limitation, the transition between these different phases is usually triggered by the accumulation of inhibitory molecules (e.g., growth inhibitors, by-products, and lysed cellular debris), ultimately limiting the overall productivity and time span of this type of process. Since 1990, product titers of discontinuous forms have increased from 0.1 g/L to 5 g/L, mainly through optimization of cell lines, media systems, process control, and switching from batch to FB mode. In addition, different strategies have been developed to increase cell productivity, such as adding specific molecules to increase productivity, increasing osmolality, and changing temperature or pH during the stabilization period so that cell activity and productivity can be sustained over a long period of time. However, the key limitations of discontinuous operation are the accumulation of cell-derived inhibitory molecules and nutrient limitations in the culture medium, which limit the productivity per unit volume of the process.
In order to bypass the unfavorable characteristics of discontinuous process forms, alternative process strategies have been sought based on uninterrupted medium replacement. These perfusion cell culture systems allow further cell proliferation by replacing the medium, thereby removing inhibitory molecules and providing fresh nutrients. Cell retention devices have been developed to continuously remove the cell-free media fraction, and the most common systems are based on alternating tangential flow (ATF) or tangential flow filtration (TFF). Pseudo-steady state can be achieved by regulating the concentration of live cells using a waste (bleeding) system to prevent nutrient limitation and concentration of cell-derived molecules, thus allowing continuous culture. As a result, perfusion cell cultures can increase productivity per unit volume and facility utilization compared to discontinuous processes. However, these continuous cultures require large amounts of media - up to several reactor volumes per day - large membrane devices for cell retention, and complex process monitoring. This increased media consumption is one of the major cost drivers in the perfusion process.
All of these mixing processes utilize filtration systems to continuously replace spent media in the reactor. However, filtration systems have shown significant drawbacks, such as slow replacement rates, which can lead to back-mixing of newly supplied media as well as membrane plugging and contamination. An emerging technology in this field is the fluidized bed centrifuge (FBC), which allows for the rapid and aseptic separation of cells from the supernatant, as well as washing and concentration under mild process conditions. The functional principle of the FBC relies on offsetting centrifugal and hydrodynamic forces resulting in cell capture while low molecular weight particles, such as spent media and mAb, can flow through. The system was originally developed as a cell clarification device and showed high recovery of mAb and cells. However, non-invasive FBC separation of cells and media was also hypothesized to be applicable in upstream processes to allow for intact media displacement intermediate processes. General concepts for returning washed cells to the cell culture system were proposed more than 10 years ago, and one of these concepts, which consisted of two bioreactors operating in parallel, in which the cell culture fluid was processed through the FBC system and transferred to the second bioreactor, has been described in a study by Jacob Arthur Tijsterman (2015). However, proof-of-concept data for these process concepts are still missing.
In this work, we present a new process scheme in which only one bioreactor is used and media replacement is applied to enhance the common replenishment batch operation. This new enhanced process concept is based on the beneficial media replacement offered by FBC and is designed to achieve higher productivity per unit volume while avoiding overuse of media and the need for extensive process control. The new process is based on an Intermediate Harvest step, in which the product is harvested after the first culture phase, while the cells are washed with fresh medium, and then returned to the same production reactor for the second production phase (Figure 1). The new intensification process was tested in a small-scale screening system to study the impact on the culture, with successive scaling up and further optimization. Overall, a scale-down option should be used to develop an intensification process with beneficial characteristics in terms of productivity and media consumption.
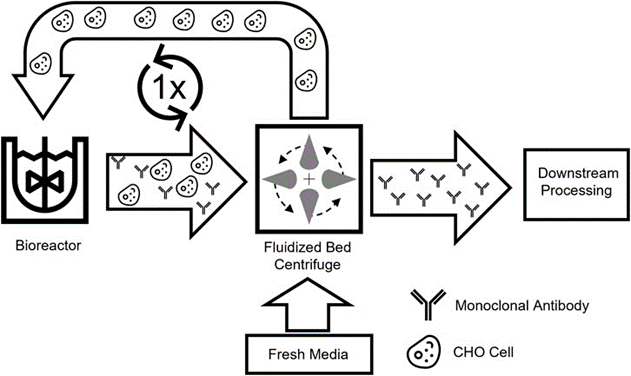
Fig. 1. Schematic representation of the intermediate harvesting process. Cell cultures containing secretions are collected from the bioreactor and separated by a disposable fluidized bed centrifuge. Afterwards, the cleaned cells can be reintroduced into the production bioreactor and used in a new process.
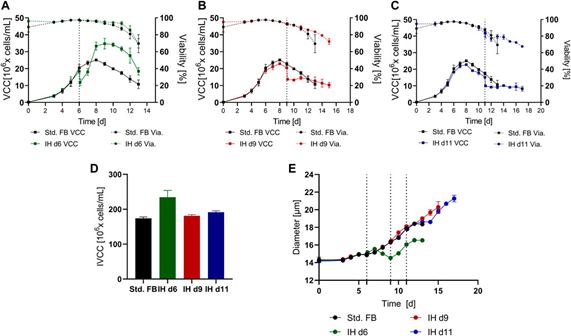
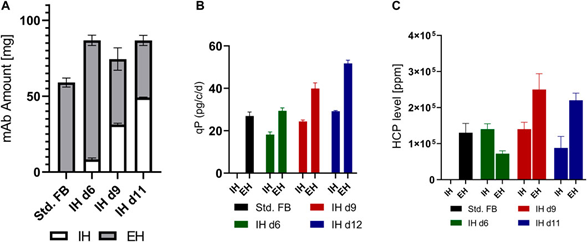
Fig. 2. Culture results of standard supplemented batch (std. FB) cultures with and without complete medium replacement at different time points over time. (A), (B), and (C) are the changes in viable cell density (VCC) and activity (Via.) at day 6 (IH d6; n = 3), day 9 (IH d9; n = 3), and day 11 (IH d11; n = 2) when medium replacement was performed as compared to standard FB (n = 3) culture, respectively. Individual time points of medium replacement are indicated by dashed lines. (D) IVCC values for all cultures throughout the incubation period. (E) Cell diameter for each culture over time.
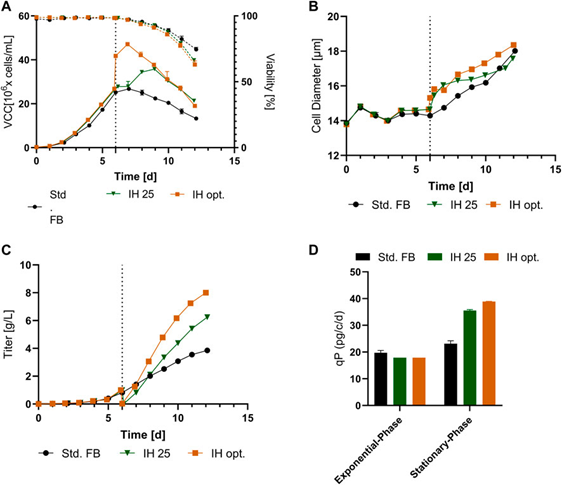
Figure 3. Supernatant analysis of small-scale screens performed at various time points in the intermediate harvest (IH) and final harvest (EH). (A) mAb accumulation for each method in the small-scale screen. (B) Average cell-specific productivity of the first culture phase prior to IH and the second phase between IH and EH (EH). Control FB productivity was calculated over the entire process duration prior to EH. (C) Ratio of HCP content to expressed mAb for each method at the time points of IH and EH.
Fig. 4. Culture results of a reduced model transferred from a 5 L reactor to 250 mL after IH operation. (A) VCC (solid line) and activity (dashed line) curves over time for standard FB, the IH method of reinoculation at 25 × 10^6 cells/mL (IH 25), and the optimized IH process (IH opt.). (B) Cell diameter changes over time for all methods. (C) mAb titers (g/L) during incubation. (D) Cell-specific productivity (qP) in pg/c/d for the exponential and stabilization phases of each experiment performed.
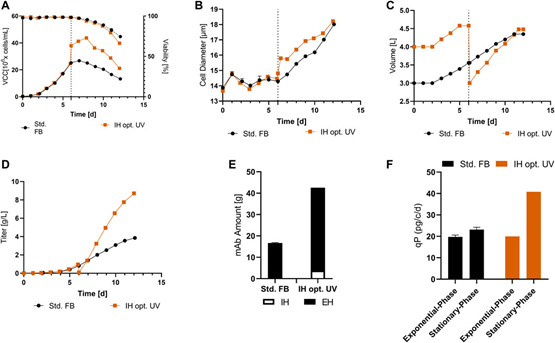
Fig. 5. Cellular and expression parameters of standard FB (n = 2) and optimized IH in UniVessel culture (IH opt.; n = 1). (A) VCC (solid line) and activity (dashed line) for both methods. (B) Diameter during incubation. (C) Volume over time for both experiments. (D) Titers (g/L) of standard FB and optimized IH over time. (E) Total amount of mAb produced by IH and EH (final harvest) in each method. (F) qP (pg/c/d) for the exponential phase (up to day 6) and the stabilization phase (from day 6 to the end) in both experiments.
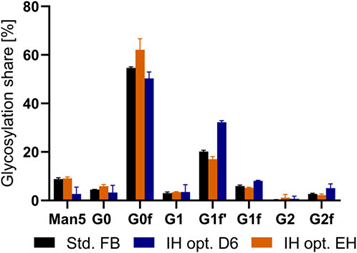
Figure 6. Percentage distribution of glycosylation of mAb expressed in the standard replenishment batch (FB) process and optimized intermediate harvest (IH opt.) on day six as well as in the final harvest (EH).
Summarize
In this study, a novel hybrid process between perfusion cell culture and classical FB culture was developed. Therefore, the effect of complete and rapid medium replacement with the FBC system (IH) during normal FB culture was investigated. Our results show two different effects on the culture depending on the time point of the IH. During the exponential growth phase, a significant prolongation of cell proliferation can be achieved, leading to higher peak cell densities. In contrast, IH during the stationary phase leads to a sustained stagnation of cell proliferation, prolonged high cellular activity, and subsequent prolongation of process duration. IH in the exponential growth phase was selected for scale-up, given the lack of significant differences in final product titers and the increased impurity/product ratio when performing IH at later time points. Feasibility as well as comparability on a small scale can be demonstrated in the scaled-up system. In addition, by increasing tank utilization and subsequently concentrating the cells after the IH operation, the process can be further optimized in subsequent steps. The results show that the new FBC-enabled process allows for a +154% mAb increase in total product yield per run compared to the standard Overall, this work demonstrates an innovative new approach to enhance commonly used FB operations without the complex integration of a perfusion cell culture system. Furthermore, due to the simplicity of the IH process, it is possible to combine this application with a variety of other enhancement methods such as high-density inoculated FB. In addition, since scale-up versions of the FBC system already exist, the established process should be fully scalable to the 2000 L scale. However, proof of concept for production scale (e.g., 2000 L) is still needed and will be the focus of future research. Overall, the presented work opens up several new possibilities to modify the current FB process to significantly increase product yield and productivity.
original text:L.N.Reger, M.Saballus, J.Matuszczyk, et al., Boosting Productivity for Advanced Biomanufacturing by Re-Using Viable Cells. Front. Bioeng. Biotechnol.,2023.






