This article is reproduced from: Instrument Information Network

Group photo of both parties
(From left to right: Li Zhaokun, Life Science Editor of Instrument Information Network;Dr Teng Xiaonuo, General Manager of Womei Biology;Kang Long, Customer Success Manager of Instrument Information Network;Fan Xuezhu, Life Science Editor of Instrument Information Network)
At present, although China's biomedical bioreactor equipment has achieved a large degree of domestic substitution, high-end products, especially ultra-large-scale bioreactors (more than 10,000 litres) are still heavily dependent on imports, which are not only costly, but also very easy to be stuck in the neck in terms of key technologies and maintenance. China in this field has long been in a state of blank, only through the localisation of tank processing, software auto-control with imports to achieve partial local substitution. In recent years, with the country's attention to the biological economy and support, China's technological innovation in the upstream biotechnology equipment and the degree of market investment continues to increase, the domestic companies have successfully developed a fully independent intellectual property rights of the domestic manufacturing, and successfully carried out the verification test of the antibody expression process. The design, manufacture and launch of this ultra-large-scale 15,000-litre bioreactor has filled the gap in this field in China.
In order to get a glimpse of this equipment, a group from Instrument Information Network went into this company, which has a good reputation for its products and services in the upstream of biomanufacturing--Suzhou Womei Biology Co.,LTD (hereinafter referred to as "Womei Biology"), a company with a reputation for upstream products and services. What is so special about this equipment? What are the difficulties that need to be overcome in the field of high-end bioreactors in China? Follow our footsteps to find out!
Focus on high-end products, successfully self-developed domestic 15,000 litres of animal cell bioreactors
As a national strategic emerging industry, high-end equipment manufacturing is an important support to enhance the core competitiveness of the manufacturing industry, achieve new industrialisation and build a strong manufacturing country. So, what is high-end equipment? High-end equipment, also known as advanced equipment, refers to advanced industrial facilities and equipment with high technological content and high value-added, which are led by high technology, are at the high-end of the value chain and the core link of the industry, and determine the comprehensive competitiveness of the entire industrial chain. Take high-end bioreactor as an example, its "high-end" embodied in the design, manufacture, operation and maintenance of various details, the system integrates such key core technologies as digital design, CFD simulation, PAT technology, QbD design concepts and intelligent control, etc., each of which needs to be supported by highly specialised talents and precision instruments as support.
"In other words, whoever masters the most advanced productivity will have an advantage in the competition. For example, in the absence of major differences in cell lines and strains, different equipment can cause a maximum difference in yield of 20-50 per cent." Dr Teng explained, "We focus on high-end products, and today, there are many companies that have started to use these high-end products, most of which are industry leaders and advanced manufacturing companies."
After Dr Teng's introduction to the concept of high-end equipment, we also walked into the Womei Bioindustrial Reactor Research and Testing Workshop. After seeing the series of bioreactor products that have gone through three iterations of technology and software, this completely self-developed 15,000-litre animal cell bioreactor (DOE-BS15000L) came into view.
This 15,000-litre animal cell bioreactor has three layers in total, and all core components are imported. When asked why he did not choose domestic parts, Dr Teng said: "The equipment is mainly used for the production of human antibody drugs, and domestic users give priority to foreign suppliers with mature regulatory certifications and a good track record of use in the parts selection stage. For the accessories of high-end reactor, the domestic accessories are not as good as the imported ones in terms of compliance, stability and key performance, which also leads to the fact that we can only make efforts to achieve the domestic substitution of the whole machine first when we carry out the domestic substitution, and for the domestic substitution of the accessories, we still need to follow up the domestic substitution with the concerted efforts of many domestic accessory vendors.
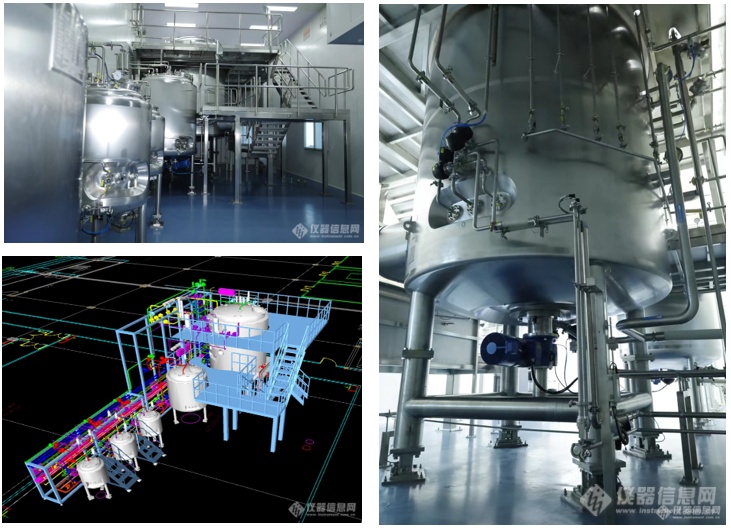
DOE series 15000L stainless steel cellular bioreactor system (DOE-BS15000L)
The layout of the industrial reactor research and testing workshop is also rationalised with full consideration of the requirements during actual GMP production. The piping gallery and the main equipment are arranged in a partitioned area, with full consideration of the operation and maintenance convenience. There are special passages for operation and maintenance, so that each fitting and each layer of piping can be easily serviced. All these equipments are fully automated and have full life cycle management function. At the same time, the whole reactor is equipped with the Raman online detection solution developed by the National Key Laboratory of Bioreactors of East China University of Science and Technology, which can realise real-time online detection of more than twenty kinds of substances including ammonia, sugar, amino acid, antibody quality attributes, and so on. Dr Teng said that the design of the reactor, the level of self-control and the PAT solution represent the highest level of current domestic bioreactors.

DOE-BS15000L's back piping distribution map
In another test lab, 35L multiplexed parallel cellular bioreactors (Figures A and B) and the fourth generation 500 ml high-throughput parallel intelligent bioreactor (Figure C) are lined up on the experimental bench, "This instrument beside me is our newly developed multiplexed high-throughput parallel intelligent bioreactor (WT-IM500), which is mainly used for High-throughput process development,, through the self-developed XBIO host computer software, it can realise one controller to manipulate the operation of 4-64 parallel bioreactors at the same time." Dr Teng introduced to us. At the same time, he believes that the key technologies of multiplexed high-throughput parallel intelligent bioreactors are mainly as follows: rational design of tanks, manufacturing and processing precision and parallelism, intelligent sensors, stable self-control system and powerful data acquisition and process analysis software. "Overall, there is no longer any obvious difference between domestic and foreign countries in terms of the technology of multi-parallel bioreactors, and there is not much difference in terms of the end-use effect. In terms of after-sales service, imported brands do far worse than domestic brands, which is one of the main reasons that prompt users to switch from imported brands to domestic brands.
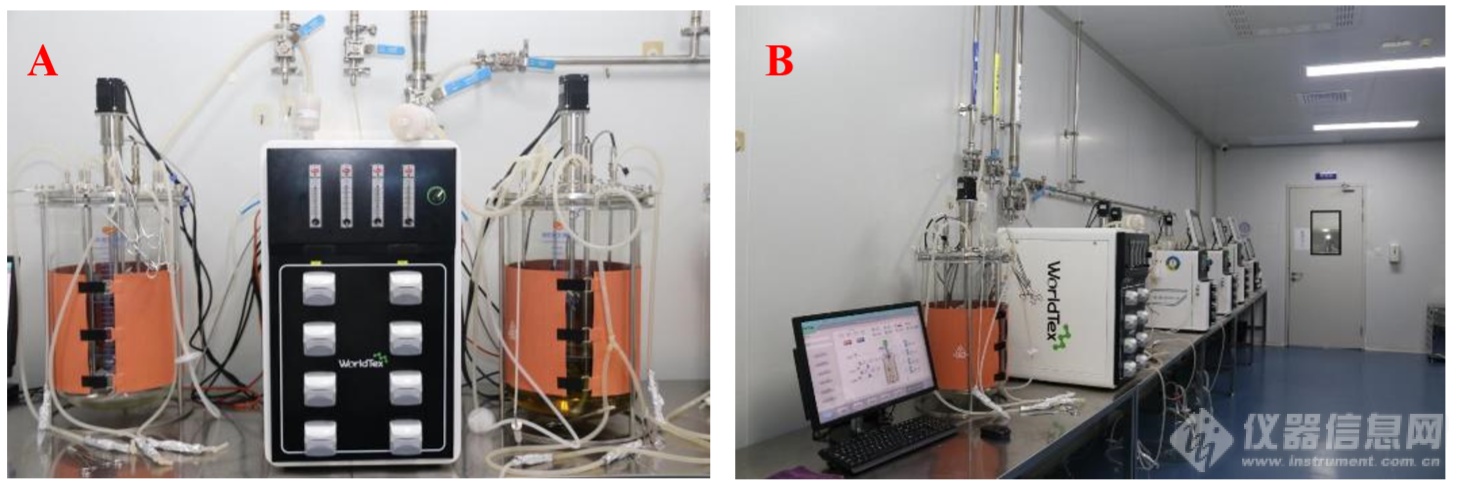
Figure A: DOE-B35 Multiple Parallel Cellular Bioreactor;Figure B: Multiple Parallel Bioreactor Products

Figure C: WT-IM500 500ml high-throughput parallel intelligent bioreactor
Relying on the technical background of East China University of Science and Technology, we have been working steadily for 15 years.
Founded in 2010, Womei Biology was established with the National Biochemical Engineering and Technology Research Centre (Shanghai) of East China University of Science and Technology (hereinafter referred to as the "Engineering Centre"). After more than a decade of development, the company has now formed a business scope around biopharmaceuticals (animal and human), synthetic biology upstream key technology services and related products, of which cell culture media and bioreactors are the company's two main product lines.
At the wall of corporate development history, Dr Teng reviewed with us the development history of Womei Biology. In the early days of the company, Womei Biology started with technical services and cell culture media in Shanghai. Thanks to the solid technical foundation, the business of Womei Biology culture media developed rapidly, and a production base was set up in Suzhou in 2012.In 2016, Womei Biology set up a bioreactor division, mainly developing various high-end bioreactors.In 2021, in order to further improve the company's equipment manufacturing capacity, Womei Biology joined hands with Pharmax Systems Engineering (Shanghai) Co Ltd (hereinafter referred to as "(hereinafter referred to as "Pharmax") to establish a joint venture company focusing on the research, development, production and sales of high-end bioreactors in one company - WorldTex (Nantong) Biotechnology Co.,LTD "), and with the East China University of Science and Technology (ECUST) State Key Experiment for Bioreactors, jointly established ECUST & VTIS Joint Technology Innovation Transfer Centre for Intelligent Manufacturing and Process Control (Innovation Centre), "through which a series of advanced key technologies and cutting-edge achievements of the most cutting-edge bioreactors can be transferred to achieve rapid industrialisation. This 15,000 litre R&D process is also strongly supported by the Innovation Centre." Dr Teng said proudly.

Dr Teng Xiaonuo introduces the development history of Womei Biology
In such a complex environment in 2024, the bioreactor segment of Womei Biology has still achieved steady development. Only in July, 16 million new orders were signed, of which more than half were from overseas. "Thanks to the country's support for the overseas market, coupled with the strong market demand in the Belt and Road countries, it has accelerated the pace of our products going out of the country." Dr Teng said.
The company has been deeply rooted in technological R&D and innovation, and has been steadily building up its business for 15 years. Nowadays, the total R&D area of Womei Biology is nearly 10,000 square metres, with the R&D centre in Shanghai as the main focus, as well as R&D centres in Suzhou Gusu District and Wuhan, and manufacturing in the Zhangjiagang plant. In addition, the Haining and Guangzhou factories of Pharmax are responsible for the main production and manufacturing of the reactors. Dr Teng said, "Currently, our manufacturing capacity, technical level and service capability are at the forefront compared with other domestic enterprises of the same type."
There is still a gap between domestic and overseas in the field of high-end bioreactors
According to Dr Teng, in the field of high-end bioreactors, the technology gaps at home and abroad, though small, still exist. These technology gaps are mainly reflected in the following aspects:
First, miniature high-throughput intelligent bioreactors. This type of instrument is crucial for synthetic biology research as well as the development of drugs and culture media, and there is no domestic enterprise that can really achieve high throughput and intelligence in this area, especially at the process level.
Second, ultra-large-scale bioreactors. Although China can currently achieve large-scale bioreactors, there is a lack of successful cases in the ultra-large-scale production and application of high-end bioreactors. Currently, China is basically dependent on imported manufacturers for 10,000 litres of cellular bioreactors, or imported technology for domestic manufacturing.
Third, the core components and sensors. In the automatic control of instrumentation, valves, core process monitoring sensors and other accessories, can not yet achieve complete localisation. Part of the field has appeared localised alternative products, but the promotion and application in the field of high-end biomedicine is still relatively scarce.
Fourth, rational design and amplification technology based on process departure. Nowadays, most of the reactors are designed to follow the example of foreign countries, "drawing on a gourd", but the real design should be combined with the characteristics of their own strains, products, processes and so on. In the amplification process, in the past, rely on experience, but the real amplification needs to rely on a full understanding of the process characteristics and equipment characteristics, flow field analysis technology plays a crucial role in solving the problem from a methodological point of view.
Synthetic biology development direction: process engineering equipment integration
Nowadays, synthetic biology has become a new business segment with fast growing performance, and its main development direction is "process-engineering-equipment integration". In the next five years, Womei Biology will continue to plough into the upstream of the biotechnology industry chain, providing not only the full-flow equipment for the process development process, but also engineering design and synthetic biology CDMO services for users. "Although the synthetic biology market has become more 'rational' this year, it is still very optimistic overall. I believe that synthetic biology will prosper for more than 50 years. As synthetic biology technology is developing rapidly, synthetic biology companies need to get their products off the ground quickly and generate benefits in order to have a future, otherwise they will easily be eliminated from the market. In the process of rapid development of enterprises, not only need the strong support of the government, but also can not be separated from the upstream enterprises of synthetic biology like us, I hope we can do a good job in the upstream prosperity, and through the upstream prosperity to support the development of the downstream."
It is reported that in addition to the East China University of Science and Technology & WorldTex Joint Technology Innovation Transfer Centre for Intelligent Manufacturing and Process Control established with the East China University of Science and Technology, Womei Biology has also cooperated with the East China University of Science and Technology to set up the Joint Technology Innovation Transfer Centre for Synthetic Biology, which focuses on the research of key synthetic biology technologies and the rapid industrialisation of scientific research results.
Talking about the future development strategy, Dr. Teng said, on the one hand, continue to increase its own R & D investment to enhance the self-blood-forming function, on the other hand, will continue to deepen the cooperation with the East China University of Science and Technology, Huazhong University of Science and Technology, Sichuan Agricultural University, Suzhou Institute of Technology and other institutions of higher learning, and accelerate the industrialisation of scientific research results through the cooperation between the university and the enterprise to ultimately achieve win-win or even a multi-win situation. In addition, Womei Biology is also actively participating in the national and local layout in the fields of biomedicine, synthetic biology, biomanufacturing, etc., and integrating its development into the national strategic development.

Dr Teng Xiaonuo, General Manager of Womei Biology






