Plasmids as Biological Drugs
The rapid growth of the gene and cell therapy industry in recent years has greatly increased the demand for plasmid DNA. Plasmids are used to deliver genetic information or genes encoding therapeutic proteins, RNAs, or antigens directly to a patient's target cells (Figure 1A and Table 1). In addition, plasmids are used as vectors to deliver molecular components of gene editing systems (e.g., editing enzymes, RNA guides), including clusters of regularly spaced short palindromic repetitive DNA sequences (CRISPR), zinc-finger nucleases (ZFNs), and transcriptional activator-like effector nucleases (TALENs). In such in vivo applications, appropriate plasmids are combined with other components (e.g., adjuvants, lipids, etc.) to produce a medicinal product for delivery to a patient. In these applications, the plasmids are biotherapeutics and must be manufactured under current Good Manufacturing Practices (cGMP) with appropriate regulation, testing and controls.
Plasmids as starting materials
In addition to their role as biologics, plasmids play a supportive role as complex starting materials in the production of engineered cell products (Figure 1Bi and Table 1) or other biologics (Figure 1Bii and Table 1). For example, in the context of chimeric antigen receptor T-cell (CAR-T) therapies, genome editing approaches, or mesenchymal stem cell therapies, plasmids are used as an alternative to viral vectors to genetically modify cells extracted from patients or donors. The first scenario entails the transfection of patient's T cells with plasmid systems (e.g., encoding CAR genes, transposases, etc.) with the aim of achieving stable gene transfer, integration and expression of CAR. In the second case, plasmids are used to deliver the molecular components of gene editors such as CRISPR, TALEN or ZFN. In either case, plasmid-modified cells are infused back into the patient. Finally, plasmids are also used to modify MSCs, for example to enhance their therapeutic function in vivo.
Plasmids are also required for the production of viral vectors and mRNAs, which can be used alone as biologics (Fig. 1Bii) or as reagents for ex vivo modification of patient cells (Fig. 1C and Table 1). For example, many adeno-associated virus (AAV) and lentiviral (LV) vectors are produced by transient transfection of production cells (e.g., human embryonic kidney (HEK) 293T cells) using multiple plasmids. As an example, the production of AAV particles relies on the use of three different plasmids: an AAV transfer plasmid flanked by two Inverted Terminal Repeat Sequences (ITRs) of the target gene; a plasmid containing the AAV gene; and a helper plasmid encoding an adenovirus helper gene. Similarly, the production of LV by transient transfection of cells requires the use of three or four different plasmids. The resulting viral vectors can then be administered to patients or used in isolated transduced cells.
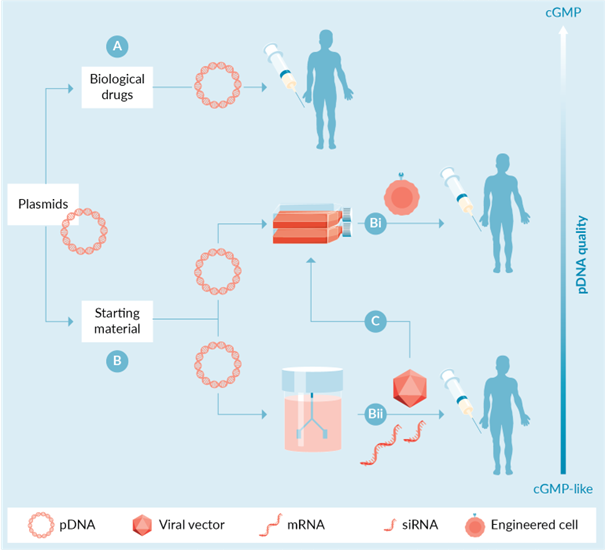
Figure 1 Direct and indirect applications of plasmid DNA in gene and cell therapy. Plasmids can be used as (A) biopharmaceuticals, e.g. as DNA vaccines or as components of in vivo non-viral gene therapy/editing platforms, or as (B) starting materials, e.g. for (Bi) ex vivo genetic engineering of cells (CAR-T cells, CRISPR-edited cells, etc.) or for the production of (Bii) viral vectors, mRNAs, and ultimately RNA biologics. The latter can be used alone as biologics (Bii) or (C) as starting material for ex vivo modification of patient cells.
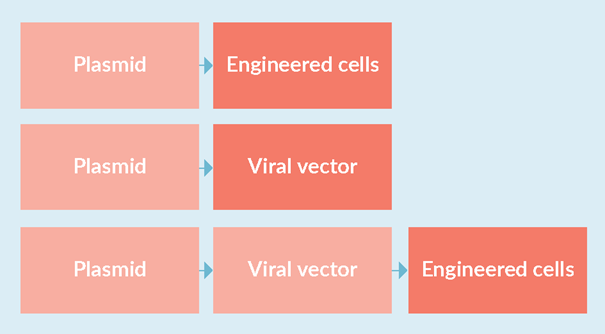
Figure 2 Preparation of plasmids as starting materials for the production of engineered cell products and viral vectors. Production activities highlighted in light red should follow cGMP principles, while those highlighted in dark red should follow full cGMP.
The emergence of mRNA vaccines triggered by the Neocoronavirus crisis has also created new uses and a surge in demand for plasmids. In the context of mRNA technology, plasmids are widely used as templates required for in vitro transcription (IVT) reactions to produce mRNA. Such templates are usually produced by enzymatic linearization of the purified plasmid or by amplification of the target region in the plasmid using PCR. mRNA products produced by IVT are then further processed and purified until the stage where they can be transferred to patients, e.g. in the context of mRNA vaccination or genome editing (Figure 1Bii and Table 1). In addition, the mRNA products can be used for ex vivo modification or editing of cells (Figure 1C and Table 1).
One can also foresee that plasmids and IVT strategies may play an even more important role in the production of small RNA molecules, such as double-stranded RNA used in antisense oligonucleotides, RNA wizards, or siRNA products.Currently, solid-phase chemical synthesis, which can generate RNAs up to 50-100 nt in length, is the method of choice for the majority of oligonucleotide drug synthesis due to its cost-effectiveness, automated scheme and very short synthesis cycle. Nevertheless, IVT is widely used to synthesize RNA molecules for structural studies and basic RNA biology (e.g., splicing, riboswitching, CRISPR, lncRNA) studies, in which case it may become an attractive alternative. If this materializes, plasmids are likely to play a key role in small RNA production, as they do in mRNA vaccines, therapeutic drugs and reagents.
Plasmid grade
The indirect use of plasmid DNA as a viral vector or starting material for mRNA vaccine production requires the production of large quantities of material. For example, more than 1 kg of plasmid DNA would be required to deliver 1 billion doses of mRNA vaccines. Since plasmids do not appear in the final drug product that is given directly to the patient, but are used as a starting material for the cGMP production of other starting materials, biopharmaceuticals, or cellular products, cGMP ratings are not strictly required. However, while not all GMP aspects or GMP certificates are required, GMP principles should be adhered to during the manufacturing process, as starting materials may ultimately end up in residues in the finished drug product and may affect its quality, safety and efficacy. Ultimately, the developer will need to conduct an appropriate risk analysis to determine the quality criteria for plasmid DNA suitable for further production of the drug product under cGMP. Relevant aspects that need to be properly considered include quality management systems, documentation, raw materials, cell banking, production, specifications, testing, control and storage. Thus, one may choose to produce a cGMP-like/high quality plasmid DNA that does not meet all cGMP requirements, but still complies with many regulatory recommendations. Figure 2 highlights the stages of production of engineered cell products and viral vectors that rely on plasmid starting materials where cGMP and cGMP principles should be applied.
Large-scale plasmid production
Despite its maturity, large-scale production of plasmid DNA is not an easy task, and manufacturers are continually forced to find ways to increase productivity without compromising quality. Part of this pressure to improve production performance stems from the fact that available capacity is insufficient to respond in a timely manner to the increased demand associated with the development of growing plasmid applications.
Currently, large-scale production of plasmid DNA relies on only one platform host - E. coli. This preference is justified by the ability of E. coli to grow and divide rapidly and provide high plasmid DNA yields under a range of conditions. In addition, there are many tools available to support the molecular and microbial engineering of E. coli, including the creation of plasmid vectors and modified strains. Improved strains of E. coli can be grown to densities of hundreds of grams per liter, producing up to 1-2 g of plasmid DNA per liter of culture, and work is also underway to develop improved strains of E. coli that can avoid instability problems, such as those faced when working with ITR-containing plasmids, such as those used in the production of AAV transfer plasmids.
One way to increase the number of plasmids generated during production, simplify regulatory approvals, and improve the biological function of plasmids is to focus on engineering the DNA backbone. There have been efforts to generate plasmids and plasmid systems (e.g., microcircuits, nanoplasmids, microcarriers) that are smaller, do not contain antibiotic resistance genes, increase yield, and provide high transgene expression.
Isolation and purification of plasmids from E. coli biomass recovered at the end of fermentation is an engineering challenge, but one that has mostly been solved, especially on a smaller scale. The series of unit operations used in plasmid downstream processes almost inevitably include base lysis, tangential flow filtration and chromatography steps. Different combinations of operations are used to process plasmid DNA with residual amounts of host impurities (genomic DNA, RNA, proteins, lipopolysaccharides, etc.) and to meet regulatory requirements.
Key issues that have not been satisfactorily addressed include poor reproducibility of alkaline lysis, lack of load and isoform selectivity in chromatography, and loss of superhelical isoforms due to shear during the process. Final decontamination filtration using 0.22 µm filters can also be cumbersome when working with very large plasmids.
Once produced, the large quantities of purified plasmids obtained from each batch should be rigorously characterized. Release specifications for plasmids used as starting materials will essentially focus on the same attributes as those covered in the production of plasmids used as biological drugs. This means that determinations of identity (e.g., sequence, homogeneity), potency (e.g., concentration, homogeneity), and purity (e.g., host impurities, bioburden, residual kanamycin), as well as corresponding acceptance criteria, must be developed.
Summarize
The demand for large-scale production of plasmids has surged in the past few years, not only because of the development of plasmid biopharmaceuticals (e.g., drugs for DNA vaccination, in vivo gene therapy, and gene editing), but also and primarily because of their supportive role in the production of many of the gene- and cell-based therapeutic products currently being produced, including viral vectors, viral vector vaccines, mRNA vaccines, microloops/microvectors/nano plasmids, and engineered cells.
In addition to current uses, it is foreseeable that plasmids may play an important role in the production of small RNA molecules (e.g., antisense oligonucleotides, RNA wizards, siRNA products) by IVT. While one might initially question whether IVT can compete with full-fledged chemical synthesis of oligonucleotides, one driver in this direction may come from an unexpected area: agriculture. Specifically, the development of novel pesticide tools based on the induction of gene silencing in plant pathogens and other pests via RNAi is driving the development of cost-effective methods for the mass production of large quantities of dsRNAs, and IVT is emerging as an alternative in this context, given that chemical synthesis is likely to be unsuitable for achieving the large-scale and low-cost production of siRNAs that is required.
Looking ahead to the plasmid production side of things, one can anticipate a number of developments that will facilitate or completely change the way plasmids are produced today. For example, while the current performance of E. coli as a plasmid production host appears to be unrivaled, one might wonder whether the high demand for plasmids might justify the search for a bacterial host with more suitable production characteristics. Gram-positive bacteria are advantageous as plasmid production hosts because they lack lipopolysaccharide, which is one of the most troublesome impurities when isolating plasmids from E. coli. Just as production hosts other than E. coli have emerged in recombinant protein production, there may be other hosts waiting to be discovered and developed into plasmid producers.
The engineering of plasmid backbones may also have an impact on the field. In addition, attention should be paid to radical innovations such as the use of minimal synthetic structures, such as "dog bones" and dumbbell-shaped DNA carriers, which are produced enzymatically. However, although these represent important advances in the field, it is highly unlikely that plasmids will become obsolete in the future. In addition, the production of small loops, microcarriers, and other minimized vectors still relies on plasmids.
Disposable technologies, process analytics, automation, digitization and continuous production are industry trends that may change the way plasmids are produced in the future. In the latter case, for example, the design of continuous cell lysis processes that are robust and capable of consistently delivering intact plasmids is another advancement to seek.
In summary, the central role that plasmids now play in the development and production of many gene and cell therapy products amply justifies a significant increase in R&D efforts and investment to improve their effectiveness and production.
original text:D.Prazeres, The supporting role of plasmids in gene & cell therapy. Cell & Gene Therapy Insights 2023; 9(5), 755–762.






