Recently, the quadrivalent influenza virus subunit vaccine (MDCK cells) researched and developed by Changchun Institute of Biological Products (Changchun Institute) has been accepted by China's National Medicines and Pharmaceutical Administration (NMPA), and it is also the fifth enterprise in China that has been accepted by the NMPA in terms of influenza vaccine MDCK cells.
Figure 1: Tetravalent Influenza Virus Subunit Vaccine (MDCK Cells) from Changchun Institute of Biological Products was accepted (Source: Center for Drug Evaluation of the State Drug Administration)
Influenza is an infectious respiratory disease caused by influenza viruses, which infects about 5% to 10% of the world's population each year. Depending on the degree and scale of prevalence, influenza epidemics can be categorized into pandemic influenza and seasonal influenza epidemics. Among them, seasonal influenza epidemics can lead to 3 to 5 million severe cases and 290,000 to 650,000 respiratory disease-related deaths globally each year, posing a serious threat to public health security.
Influenza vaccine is the most effective way to prevent influenza. Since the establishment of the MDCK cell line in the late 1950s, the fact that influenza viruses can be cultured in a variety of cells, including MDCK cells and Vero cells, has been revealed, and the substrate for the manufacture of influenza vaccines has gradually begun to change from chicken embryos to cells.
MDCK cells are a wall culture cell line established by Madin and Darby in 1958 from canine kidney tissue. Compared with other cell lines, influenza virus replicates more rapidly in MDCK cells, which can be rapidly adapted to obtain high-yield virus strains within 3-10 generations, thus shortening the time for the production of vaccine strains, and at the same time, the reduction of the number of adapted generations can reduce the possibility of the occurrence of adaptive mutations of the vaccine strains in the process of transmitting the vaccine strains.
Thus MDCK cells became recognized as one of the most important cell lines most suitable for influenza virus strain isolation and vaccine production. In addition, this cell line is more easily adapted to serum-free medium, as well as serum-free suspension domestication, which is conducive to large-scale industrial production.
The culture medium used for the NMPA-accepted quadrivalent influenza virus subunit vaccine (MDCK cells) of Changchun Institute is the MDCK serum-free culture medium developed by Suzhou Womei Biological Co. The product is serum-free, protein-free and without animal source components.
Womei Bio MDCK Medium supports MDCK cell suspension culture, eliminates the problem of cell damage caused by amplification during microcarrier suspension culture, solves the limitation of cell density during large-scale microcarrier suspension culture, and increases cell density, improves the efficiency of viral amplification, and makes it easy to realize the amplification from laboratory scale to large-scale production.

Figure 2: MDCK serum free medium
I. Product characteristics:
●serum-free
●No animal sources
●No Protein Ingredients
●Supports rapid serum-free suspension adaptation of adherent MDCK
●Supports efficient proliferation and high-density culture of MDCK cells
●Supports efficient amplification of human influenza virus
II. Product performance:
1, MS01A cell culture medium is a serum-free medium, which can support high-density cell expansion and supports the addition of trypsin and specimen directly to the cell suspension for influenza virus isolation and proliferation.MDCK cells were cultured in suspension, and the highest cell density could reach more than 1.5×107cells/ml, see Figure 3.
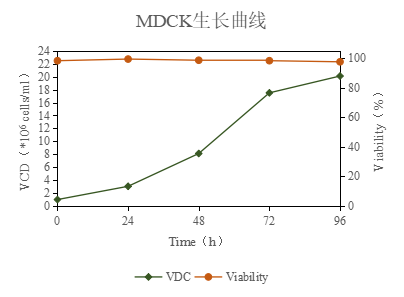
Figure 3: Growth curve of MDCK serum free medium
2、cell proliferation is stable, can be continuous high-density dilution transmission, 48h density are stable at 1.0 × 107cells/ml or more; cell state is good, permeable round, see Figure 4.
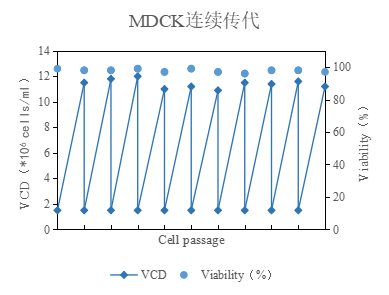
Figure 4: Successive multi-generation passaging data of MDCK cells
3、Influenza (H₁, H₃ and H₅ sub-strains) hemagglutination HA potency can be stabilized greater than 10log₂HAU/50ul, up to 12log₂HAU/50ul.
III. Ordering information:
Pseudolaric acid | Product Number | Product Specification |
MS01A | MS41104-2 | 10L/bag |
MS01A(T) | MS41104T-2 | 10L/bag |
MS01 (Industrial) | MS41204-2 | 1L/bottle |
Please use the contact information below to request a trial
Technical/Sales Service Hotline:0512-58880188
Email:womeishengwu@szwmbio.com






